International Journal of Pharmaceutical Sciences and Developmental Research
Generation of Porous Structure from Basil Seed Mucilage via Supercritical Fluid Assisted Process for Biomedical Applications
Iman Akbari and Seyyed Mohammad Ghoreishi*
Cite this as
Akbari I, Ghoreishi SM (2017) Generation of Porous Structure from Basil Seed Mucilage via Supercritical Fluid Assisted Process for Biomedical Applications. Int J Pharm Sci Dev Res 3(1): 030-035. DOI: 10.17352/ijpsdr.000014Mucilage’s are plant derived natural polymer which are valuable due to their nontoxicity, low cost and nonirritating nature, with wide range of applications. In this work, extracted mucilage from basil seeds (BSM) was dried using three various drying methods including (1) laboratory oven drying (2) water substitution with organic solvent and laboratory oven drying and (3) water substitution with organic solvent and supercritical carbon dioxide (SC-CO2) gel drying process. The obtained products were characterized by SEM, BET and FTIR and were compared both qualitatively and quantitatively. The results of this study show that, using SC-CO2 assisted process, the 3-D BSM nanostructured networks were obtained with the pores size diameter about 40 nm, without any agglomeration. Furthermore, specific area of the final products was increased from 69 to 92 m2/g by SC-CO2 gel drying in compression with air gel drying. Our observations show that, the amount of solvent residual in the SC-CO2 dried product was affected by the weight of sample, CO2 addition rate and drying time. The residual amount of organic solvent (ethanol) for CO2 flow rate of 2 mL/min was found to be 8 ppm after 90 min drying time. The FTIR analyses indicated that the nature of final product did not change during supercritical drying procedure. Overall, the ability to form 3-D structures, and bio adhesive property make BSM as a suitable low cost polysaccharide for biomedical applications such as drug delivery medium, wound dressing and also tissue engineering.
Introduction
In recent years, natural polymers have been investigated and employed for technological and industrial-related applications. Nowadays, a lot of the research effort in the biomedical field is based on natural polymers [1-3]. These natural materials have advantages over synthetic ones since they are chemically inert, nontoxic, less expensive, biodegradable, and widely available [4]. Furthermore, owing to their similarity with the extracellular matrix (ECM), natural polymers may also avoid the stimulation of chronic inflammation or immunological reactions and toxicity, often detected with synthetic polymers [5]. Mucilage’s are plant derived natural polymers which are well known since ancient times for their medicinal use. Acacia, tragacanth, gum ghatti, gum karaya, guar gum, and ocimum bascilium are popular examples of plant mucilage’s with a wide range of pharmaceutical applications [4,6,7]. Nowadays, mucilages are widely used in the pharmaceutical industries as thickeners, suspending agents, binders, etc. [8].
Ocimum basilicum L. also known as basil is a common herb plant found in many parts of the world especially in the tropical regions of Asia, Africa and central and South America well known since ancient times for their medicinal use [9]. Basil seeds have been used in traditional medicine for a long time to treat colic ulcer, dyspepsia, diarrhea and inflammations, among others ailments [10]. Basil seeds are black in color and oval in shape and due to the presence of a polysaccharide layer, when they are soaked in water, the outer pericarp swells into mucilage which could be extracted from seeds and dried or concentrated for further applications.
Pharmaceutical applications of various natural mucilage’s and their modified forms were reviewed by Prajapati et al. [8]. In the Nerkar and Gattani study, buckle mucoadhesive gel using cress seed mucilage was prepared and the diffusion of venlafaxine from obtained gel was evaluated [11]. Almeida et al., studied the rheological and thermal properties of hydrogel mixtures containing xanthan gum, konjac gum, iota-carrageenan, and kappa-carrageenan for skin scaffold applications [12]. Oliviera et al., were analyzed gellan gum hydrogels for cartilage tissue engineering applications [13]. In our recently published work, extracted mucilage from basil seeds was dried using supercritical CO2 (SC-CO2) to form a 2-D nonporous structure for pharmaceutical applications [14]. In the mentioned work, drying procedure was performed using a modified method based on Temtem et al. [15] study in which methanol was used as co-solvent to improve water solubility in SC-CO2. In the other previous work, ocimum basilicum mucilage was employed as a cost effective polymeric medium for precipitation of paclitaxel for controlled drug delivery [14].
In this work, extracted mucilage from basil seeds was dried using supercritical CO2 (SC-CO2) to form a 3-D nonporous structure 64 for pharmaceutical applications such as tissue replacing applications and drug delivery matrices. A wide variety of technologies are now available to fabricate 3-D scaffolds. Most of previous techniques proposed for gel drying present some limitations such as time-consuming, presence of organic solvents, that are difficult to be eliminated and that can remain entrapped inside the polymeric network, limitation in the obtainment and preservation of various levels of porosity and the three-dimensional structure. To overcome these limitations, SC-CO2 assisted processes have been recently implemented. SC-CO2 has been used as an alternative non-solvent in phase inversion processes to generate polymeric and bio polymeric porous structure [16-18]. Although, the obtained structures have a good interconnectivity and high porosity, but it is very difficult to obtain complex 3-D structures (flat membranes are usually generated. However, SC-CO2 shows a very limited compatibility with polar components, like water, at the ordinary temperatures and pressures used in SC-CO2 processing; for example, at 40 °C and 100 bar, water solubility is around 0.5% [19]. Thus, the common supercritical gel drying process is not directly applicable to polymeric hydrogels and a water/solvent substitution method is required. Therefore a new SC-CO2 assisted process for the production of poly (L-lactic acid) (PLLA) scaffolds has been proposed by Reverchon et al. [20]). In this work, the drying procedure was performed using mentioned method in which a water/solvent substitution step is added to the process [20,21]. The obtained nonporous product was also characterized from a macroscopic and microscopic point of view using SEM imaging and also surface area measuring. The Fourier transforms infrared spectroscopy (FTIR) analyses were performed to determine the suitability of SC-CO2 process to produce unchanged structure. Furthermore, the effect of drying time and CO2 flow rate on the solvent residue was determined.
Materials and Methods
Materials
Basil seeds used in this study were purchased from a local market in Isfahan, Iran. Ethanol and acetone (99.99% purity) was obtained from Merck and industrial grade carbon dioxide (≥ 99.9%) was purchased from Zamzam Co. (Isfahan, Iran). All materials were used as received.
Mucilage extraction procedure
The procedure of mucilage extraction from basil seeds was described in details in our previous work [15]. As stated in the mentioned study, the basil seeds were completely swelled in distilled water. The swelled seeds were washed with ethanol/water solution. Seeds solution was passed through an extractor (Pars Khazar 700P, Rasht, Iran) to scrape the mucilage layer off the seed surface. The separated mucilage was passed through a vacuum filter to remove all likely seed residuals. In order to remove protein and ash content, after filtration of the crude extract, pure ethanol was added to the extracted mucilage in the 3: 1 ratio and left overnight at 4 °C. Finally, crude extract concentrated at 55 °C with rotary vacuum evaporator (IKA, RV 10, and Deutschland, Germany) to remove extra water/ethanol content and then dried in a laboratory oven to produce dry BSM powder. Three samples of the BSM thick gel were prepared by dissolving 5 mg of dried BSM powder in 1 mL of pure water and each sample was dried in the following manner: The first sample, the reference, was dried with air in a laboratory oven at 50 °C for 6 h. The samples No. 2 and 3 were put in a bath of ethanol at -20 °C for 24 h, to allow substitution of water with ethanol, and were dried in a16 laboratory oven at 50 °C for 4 h and by SC-CO2, respectively.
SC-CO2 drying experimental set-up
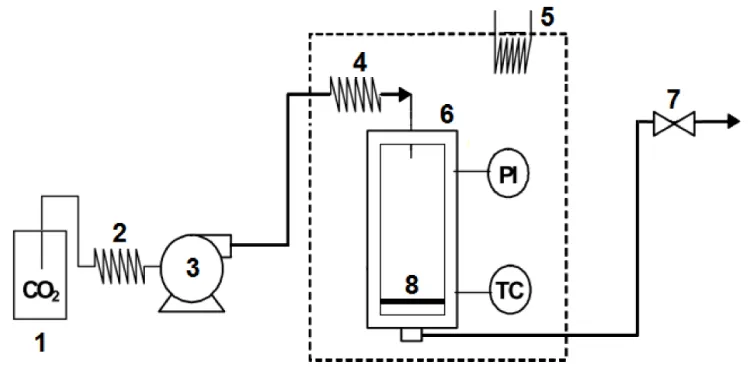
The employed experimental apparatus in this work is similar to our previous works [14,22,23]. Figure 1 shows a scheme of the experimental set-up. Liquid carbon dioxide was drawn from the lower bottom of the cylinder (1) and then sub-cooled in a cooler (2). Sub cooled carbon dioxide was pumped with a high pressure pump (3) (Jasco–Milroyal B, France). High pressure carbon dioxide was heated in a spiral heat exchanger (4) located in an oven (5) before charging into the 15 ml stainless steel high pressure vessel (6). The pressure was controlled by a back pressure regulator (7) (Tescom, elk river, USA). A metallic porous disc (8) was located at bottom of the vessel which 126 mucilage samples were placed on it to be dried.
Supercritical gel drying procedure
SC-CO2 gel drying was performed according to the following procedure: 5 mg of dried BSM powder was dissolved in 1 mL of distillated water to form thick BSM gel. The obtained thick gel kept in a bath of ethanol for 24 h and at - 20 °C. The sample put on the porous disc located at the bottom of the 316 stainless steel high-pressure vessel. The vessel was closed; 10 ml of pure ethanol was charged to the vessel and then filled from the top with supercritical CO2. When the required pressure and temperature were obtained (220-240 bar and 35-45 °C), drying was performed with a SC-CO2 flow rate of 1, 2 and 3 mL/min. The drying time lasted in the range of 40-90 min. The vessel depressurized for about 10 min to bring back the system to atmospheric pressure.
Scanning electron microscopy characterizations
To investigate microscopic structure of products, scanning electron microscopy (SEM) was utilized. The samples were sputter coated with gold (SC 7620, Quorum Technologies, UK) at 30 mA for 180 s and analyzed by a scanning electron microscope (DSM 960A, Carl Zeiss AG, Germany) to evidence the micro- and nanostructure and to measure the diameter of the pores and fibers forming the structure.
Surface area characterization
Surface areas were determined from the isotherm of adsorption of liquid nitrogen using the Brunauer–Emmett–Teller (BET) method (measured on a Quantachrome ChemBET 3000, Florida, and USA, equipped with a thermal conductivity detector) at degasing temperature of 60 °C.
FTIR characterization
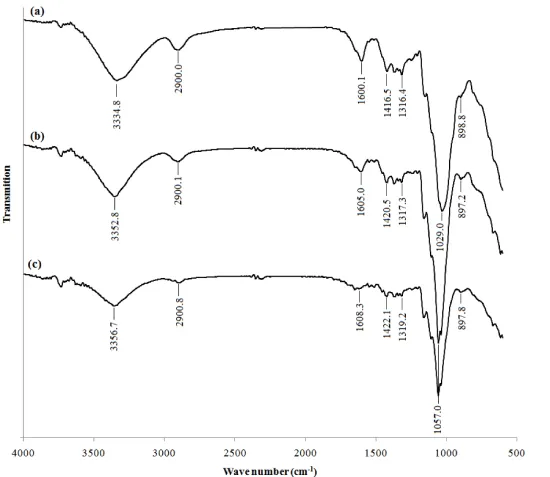
The FTIR spectra are considered as useful information for partial characterization of seaweeds [24]. In this work, FTIR (Tensor 27; Bruker) analysis was used to characterize the chemical structure of BSM. Spectra were taken in the region between 4000 and 500 cm−1. Furthermore the suitability of SC-CO2 process to produce unchanged structure was determines using FTIR spectra.
Solvent residue analysis
After carrying out each SC-CO2 drying procedure at specified time, the dried sample was removed from the vessel and the amount of organic solvent (ethanol) residue was measured using an Agilent HP-6890N gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with HP-5 5% phenyl methyl siloxane capillary column (30m~320μm~0.25μm, nominal) equipped with a G1540N-210 FID detector.
Results and Discussion
In this work, supercritical gel drying was utilized to generate nonporous structure from mucilaginous seeds of ocimum basilicum for tissue engineering applications. Our attention was focused on and the difference between obtained structure in three various drying methods including (1) no water substitution and laboratory oven drying (2) water substitution and laboratory oven drying (3) water substitution and supercritical gel drying. Furthermore, FTIR analyses were performed to determine the chemical structures of the BSM and also identify the effect of drying procedure on the final products structure.
Laboratory oven drying of virgin sample
For the reference sample, hydrogel (with water to dried mucilage ratio of 200) with no further treatment was put on the stainless steel plate and dried in laboratory oven at 50 °C. As expected, the final product demonstrated a 2-dimensional membrane (Figure 2) which its SEM image (Figure 3) shows non-porous structure. The solid sphere formed on plate surface is because of gel coagulation and impurities.
Water substitution and laboratory oven drying
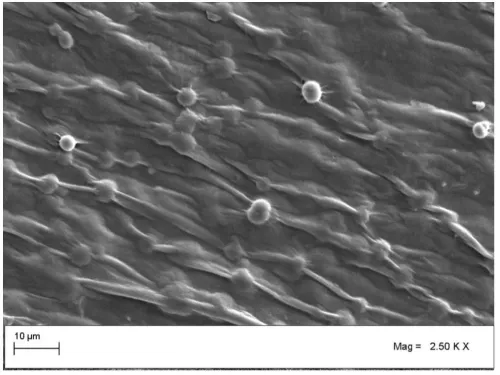
The next sample was prepared by water substitution and drying in laboratory oven. To this purpose extra water content of gel was substituted by ethanol via putting in bath for 24 h at -20 °C and dried in laboratory oven at 50 °C. The substitution of water with an organic solvent is one of the most common procedures used for the drying of hydrogels [25]. Ethanol and acetone were tested as water substituting agent. Since during ethanol substitution a complete preservation of gel shape and size was observed in contrast to acetone substitution, thus, ethanol was selected as water substituent. In water substitution step, gel precipitated in ethanol bath as a 3-D structure, thus, the final product from sample No. 2 was not obtained as flat structure like the first sample. Nevertheless, in this case, the initial 3-D shape was partly shrined due to drying and subsequently a size reduction of final structure was observed (Figure 4). SEM images shown in figure 4 confirm the partial collapse of the structure. As demonstrated in figure 5, porous structure with minor porosity was obtained. Specific surface area of obtained sample was measured using BET method. The result showed a value of 69 m2/g. Micro size filaments in this structure are result of water substitution step, in which gel precipitated in the ethanol bath and forms filament.
Water substitution followed by supercritical gel drying
In order to overcome collapsing of precipitated filaments, and to obtain nonporous structure with high porosity, after substitution of water with organic solvent, drying procedure was carried out using SC-CO2, in this part of work. The water substitution with an organic solvent readily soluble in SC-CO2 eliminated the solubility problem of water in CO2. The same results were reported in processing of chitosan [21] and PLLA [20].
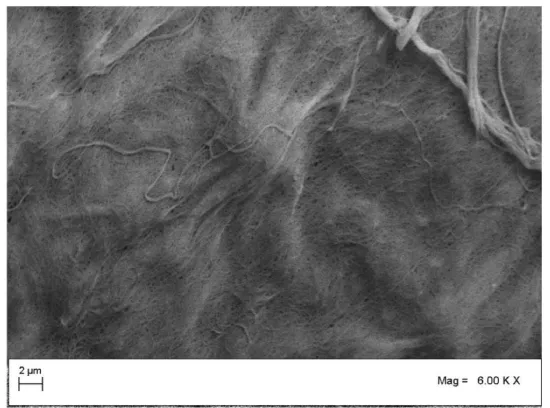
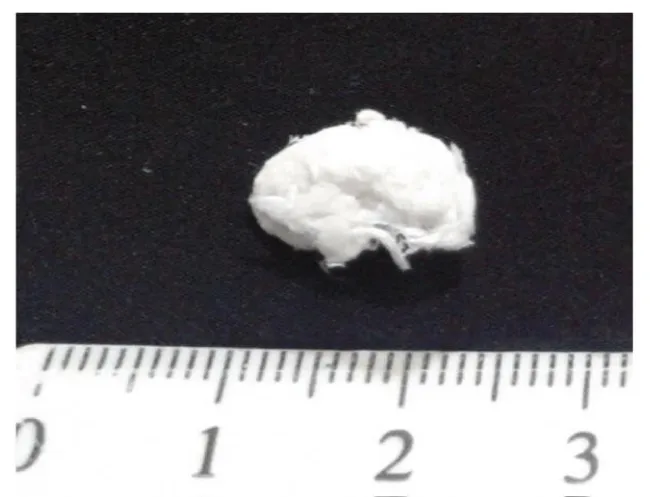
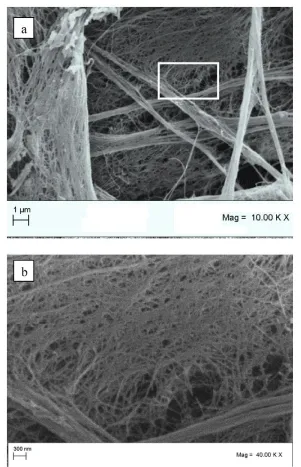
The obtained hydrogel was precipitated in pure ethanol and centrifuged (5000 rpm, 10 min at 4 °C) to remove extra water/ethanol content. Resulted thick gel in a bath of ethanol was dried using SC-CO2 as mentioned in 2.2 (Mucilage extraction procedure). Processing temperature was set in regard to the CO2 critical temperature (T=304.2 K) and the possible gum thermal degradation. Processing pressure was selected to assure a sufficient SC-CO2 solvent power for ethanol/water mixture. The drying temperature and pressure were kept constant during the experimentation. The required flushing time to ensure that the obtained final products were free of solvent, water and ethanol, was different for each experiment. Initial investigations indicated that, for all cases, flushing time of 2 h was a reasonable and sufficient time to obtain free solvent (ethanol/water) products. Thus, flushing time of 2 hours was selected as the optimum for all experiments. After flushing time, the dried BSM was taken from the vessel and was analyzed by SEM, BET and FTIR. In this case, the 3-D shape and the sample size not only was successfully preserved, but also increased in size due to diffusion of SC-CO2 in gel structure (Figure 6). The reported SEM images in figure 7 show the presence of micro porous structure with a uniform sub nonmetric network. The mean diameter of the nano metric fibers was measured to be about 40 nm. Specific surface areas of obtained samples were measured using BET method. The results showed a value of 92 m2/g which was increased more than 30 percent and indicate high porosity of the obtained sample.
In the SC-CO2 process, volume expansion of solution occurs when the CO2 is dissolved in the solution as modelled in our previous work thermodynamically [23]. The volume expansion of solution, water and ethanol in this case, leads to expansion of polymer structure and thus high level of porosity was obtained using SC-CO2 gel drying method in contrast to oven drying method. Furthermore, during the expansion, because of high diffusivity, supercritical mixture diffuse to the polymer structure and thus nonmetric fibers and pores were obtained. The biocompatible scaffold obtained by this method demonstrated a high level of porosity, with a good interconnectivity among the pore network system. The obtained structure has a high level of porosity, with a good interconnectivity among the pore network system. Thus, the obtained structures present morphology very similar to an extracellular matrix (ECM), i.e. a finely interconnected nonmetric substructure, suitable for scaffolding applications in tissue engineering. Furthermore the obtained scaffold provides support for cell attachment continued by their proliferation and differentiation and also allowing controlled release of biomaterial exposed to cells.
Solvent residual
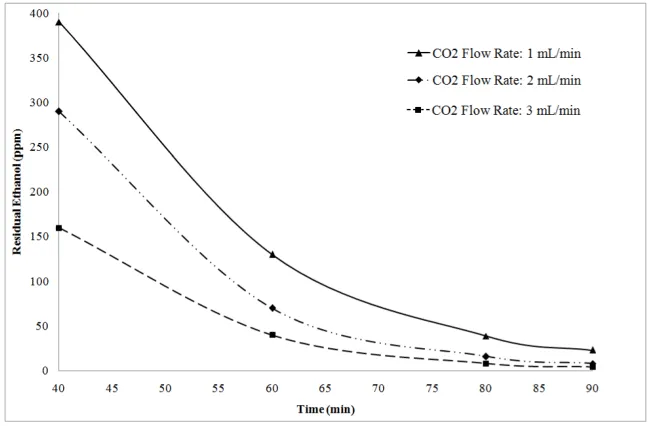
The results of this study indicated that the amount of solvent residual in the SC-CO2 dried product was affected by the weight of sample, CO2 addition rate and drying time. Figure 8 shows residual amount of ethanol in the final product versus drying time for 5 mg of dried sample and for CO2 flow rates of 1, 2 and 3 mL/min. According to figure 8, as CO2 flow rate increased, ethanol residual decreased. As drying time increases, the CO2 diffusion to the polymeric network becomes the controlling step in the drying process and its effect governs the solvent residual amount. Thus, the solvent residual reduction at higher drying time (75-90 min) reaches an almost steady state trend despite of higher CO2 flow rate. The residual amount of ethanol for CO2 flow rate of 2 mL/min was found to be 8 ppm after 90 min drying.
FTIR characterization of the obtained products
The FTIR spectra are considered as useful information for partial characterization of seaweeds [24]. In our previous work FTIR spectra of BSM was discussed in details. As shown in figure 9, FTIR spectra of three final products show same peaks which indicate that the structure of the BSM was not affected by the drying procedures. Furthermore, as mentioned in the previous work and the obtained peaks of figure 9 indicated that, BSM has good bio adhesive property and thus this porous polysaccharide can be employed formation for tissue engineering and wound dressing applications. Many actives can be released through such bio adhesives, as steroids, anti-inflammatory agents, pH sensitive peptides and small proteins such as insulin, and local treatments to alleviate pain in the buckle cavity.
Conclusions
The generation of polymeric nonmetric structured from mucilaginous seeds of ocimum basilicum was successfully carried out for scaffolding applications using SC-CO2 based methods for hydrogels drying. Various drying methods were used and finally an efficient low temperature water–solvent substitution step followed by SC-CO2 drying was developed in order to obtain a nonporous structure. It was shown that a collapsed structure with minor porosity was obtained by using water/solvent substitution and oven drying, while a nonmetric fibrous network was achieved by the SC-CO2 assisted process. The results of IR data show that BSM has good bio adhesive property and many actives can be released through such bio adhesives, as steroids, anti-inflammatory agents, pH sensitive peptides and small proteins such as insulin, and local treatments to alleviate pain in the buckle cavity. Furthermore, the amount of organic solvent residual in the final product could be controlled by varying CO2 flow rate and drying time. Overall, the synthesized nonporous structured natural polymeric material of this study could be employed for biomedical applications including tissue engineering, drug delivery and post-surgical application.
- Alves A, Caridade SG, Mano JF, Sousa RA, Reis RL (2010) Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr Res 345: 2194-2200. Link: https://goo.gl/f9NOHY
- Gomes ME, Azevedo HS, Moreira A, Ellä V, Kellomäki M, et al. (2008) Starch–poly (ε‐caprolactone) and starch–poly (lactic acid) fibre‐mesh scaffolds for bone tissue engineering applications: structure, mechanical properties and degradation behaviour. J Tissue Eng Regen 2: 243-252. Link: https://goo.gl/vgKsqv
- Silva SS, Maniglio D, Motta A, Mano JF, Reis RL, et al. (2008) Genipin‐Modified Silk‐Fibroin Nanometric Nets. Macromol Biosci 8: 766-774. Link: https://goo.gl/71cLIF
- Meka VS, Nali SR, Songa AS, Kolapalli VRM (2012) Characterization and in vitro drug release studies of a natural polysaccharide Terminalia catappa Gum (Badam Gum). AAPS Pharm SciTech 13: 1451-1464. Link: https://goo.gl/FzfVXc
- Mano J, Silva G, Azevedo HS, Malafaya P, Sousa R, et al. (2007) Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J Royal Soc Interface 4: 999-1030. Link: https://goo.gl/xUx8L9
- Das A, Wadhwa S, Srivastava A (2006) Cross-linked guar gum hydrogel discs for colon-specific delivery of ibuprofen: formulation and in vitro evaluation. Drug Deliv 13: 139-142. Link: https://goo.gl/4MAo4e
- Manikoth R, Kanungo I, Fathima NN, Rao JR (2012) Dielectric behavior and pore size distribution of collagen–guar gum composites: Effect of guar gum. Carbohydr Polym 88: 628-637. Link: https://goo.gl/2r0mab
- Prajapati VD, Jani GK, Moradiya NG, Randeria NP (2013) Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr Polym 92: 1685-1699. Link: https://goo.gl/xl92Mv
- Archana G, Sabina K, Babuskin S, Radhakrishnan K, Sukumar M, et al. (2013) Preparation and characterization of Mucilage polysaccharide for biomedical applications. Carbohydrate Polymers 98: 89-94. Link: https://goo.gl/p4ljj8
- Hosseini-Parvar S, Matia-Merino L, Goh K, Razavi S, Mortazavi S (2010) Steady shear flow behavior of gum extracted from Ocimum basilicum L. seed: Effect of concentration and temperature. J Food Eng 101: 236-243. Link: https://goo.gl/ZW72Xm
- Nerkar PP, Gattani SG (2012) Cress seed mucilage based buccal mucoadhesive gel of venlafaxine: in vivo, in vitro evaluation. J Mater Sci: Mater Med 23: 771-779. Link: https://goo.gl/pTnZhQ
- Almeida N, Mueller A, Hirschi S, Rakesh L (2014) Rheological studies of polysaccharides forvskin scaffolds. Journal of Biomedical Materials Research Part A 102: 1510-1517. Link: https://goo.gl/jyBWaz
- Oliveira JT, Martins L, Picciochi R, Malafaya P, Sousa R, et al. (2010) Gellan gum: a new biomaterial for cartilage tissue engineering applications. Journal of biomedical materials research Part A 93: 852-863. Link: https://goo.gl/PNCizs
- Akbari I, Ghoreishi SM, Habibi N (2014) Generation and precipitation of paclitaxel nanoparticles in basil seed mucilage via combination of supercritical gas ant solvent and phase inversion techniques. Journal of Supercritical Fluids 94: 182-188. Link: https://goo.gl/6ygkEH
- Akbari I, Ghoreishi SM, Habibi N (2015) Supercritical CO2 Generation of Nano metric Structure from Ocimum basilicum Mucilage Prepared for Pharmaceutical Applications. AAPS Pharm SciTech 16: 428-434. Link: https://goo.gl/Yqau8l
- Temtem M, Silva L, Andrade PZ, dos Santos F, da Silva CL, et al. (2009) Supercritical CO2 generating chitosan devices with controlled morphology. Potential application for drug delivery and mesenchymal stem cell culture. J Superscript Fluids 48: 269-277. Link: https://goo.gl/uI6O8w
- Reverchon E, Cardea S (2005) Formation of polysulfone membranes by supercritical CO2. J Superscript Fluids 35: 140-146. Link: https://goo.gl/sX56Dc
- Tsivintzelis I, Pavlidou E, Panayiotou C (2007) Porous scaffolds prepared by phase inversion using supercritical CO2 as antisolvent: I. Poly (l-lactic acid). J Superscript Fluids 40: 317-322. Link: https://goo.gl/Nqpd24
- Sabirzyanov A, Akhunov A, Gumerov F (2002) Solubility of water in supercritical carbon dioxide. High Temperature 40: 203-206. Link: https://goo.gl/SgB7zT
- Reverchon E, Pisanti P, Cardea S (2009) Nanostructured PLLA− Hydroxyapatite Scaffolds Produced by a Supercritical Assisted Technique. Ind Eng Chem Res 48: 5310-5316. Link: https://goo.gl/VuJpWz
- Cardea S, Pisanti P, Reverchon E (2010) Generation of chitosan nanoporous structures for tissue engineering applications using a supercritical fluid assisted process. J Supercrit Fluids 54: 290-295. Link: https://goo.gl/lISqOJ
- Esfandiari N, Ghoreishi SM (2013) Synthesis of 5-Fluorouracil nanoparticles via supercritical gas antisolvent process. J Supercrit Fluids 84: 205-210. Link: https://goo.gl/Z5pXNa
- Esfandiari N, Ghoreishi SM (2013) Kinetics modeling of ampicillin nanoparticles synthesis via supercritical gas antisolvent process. J Supercrit Fluids 81: 119–127. Link: https://goo.gl/X6bEyx
- Madhaiyan B, Rajasulochana N (2012) Chemical and Spectral Characterization of Cell Wall Polysaccharide of Gracilaria edulis (Gmelin) Silva. Journal of Theoretical and Experimental Biology 8: 147-151. Link: https://goo.gl/9zhdCN
- Zhang R, Li W, Li K, Lu C, Zhan L, et al. (2004) Effect of concentration of reactants on porosity of hydrogels, organic and carbon aerogels. Microporous Mesoporous Mater 72: 167-173. Link: https://goo.gl/NZkPZe
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
