International Journal of Pharmaceutical Sciences and Developmental Research
Visualization of the obscure inhalation stage in inhalation therapy
Hiroyuki Ohbayashi*
Cite this as
Ohbayashi H (2023) Visualization of the obscure inhalation stage in inhalation therapy. Int J Pharm Sci Dev Res 9(1): 028-032. DOI: 10.17352/ijpsdr.000049Copyright License
© 2023 Ohbayashi H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The flow of the inhalation procedure during inhalation therapy can be divided into three successive stages: a pre-inhalation preparatory stage, a drug inhalation stage, and a post-inhalation stage. Among these, the second stage, drug inhalation, is the most important and obscure. Using ambiguous verbal expression, the drug inhalation method is communicated to the patient using terms such as strongly, deeply, and slowly. Patients usually determine their optimal method of drug inhalation device independently, based on their own interpretation and understanding of the verbal instructions. This may make the precise inhalation using an inhalation device unpredictable. The Tokico Inhalation Monitor TM (TIM) was developed to resolve the unpredictability of this second (drug inhalation) stage. The TIM can simultaneously measure the inhalation flow rate, duration, and total volume, and display them on the screen in real-time. This mini-review demonstrates the effects of inhalation instruction using TIM, which allows the second stage of therapy, drug inhalation, to be displayed on a screen in real-time.
Abbreviations
COPD: Chronic Obstructive Pulmonary Disease; pMDI: pressurized Metered-Dose Inhalers; AUC: Area under the Flow Rate-Time Curve; FIR: Flow Increase Rate; PFR: Peak Flow Rate; TIM: Tokico Inhalation MonitorTM
Introduction
Inhalation therapy is an effective and reasonable method in which a drug is administered directly to a disease-causing lesion. It is therefore the recommended method of treatment in major treatment guidelines for asthma, Chronic Obstructive Pulmonary Disease (COPD), chronic inflammatory diseases, and tropical lung diseases [1,2]. However, in inhalation therapy, the convenience of other treatment types is lost. One of the most significant differences between inhalation and conventional oral therapies is the presence of a dedicated inhalation device that stores the drug and assists with inhalation during drug administration. Inhalation devices make the process of drug administration more confusing and laborious than that of oral medications [3]. This increases the need for education on inhalation therapy for patients in clinical settings. Nevertheless, many patients are not trained to inhale mastery in real-world practice; furthermore, even where mastery is initially achieved, ongoing assessment and education are seldom maintained [4]. The drug inhalation method is often communicated to the patient using verbal expression. However, this may make patients’ own interpretation and misleading of inhalation methods. This mini-review aimed to demonstrate the effects of inhalation instruction using the Tokico Inhalation MonitorTM (TIM), which allows the second stage of therapy, drug inhalation, to be displayed on a screen in real-time.
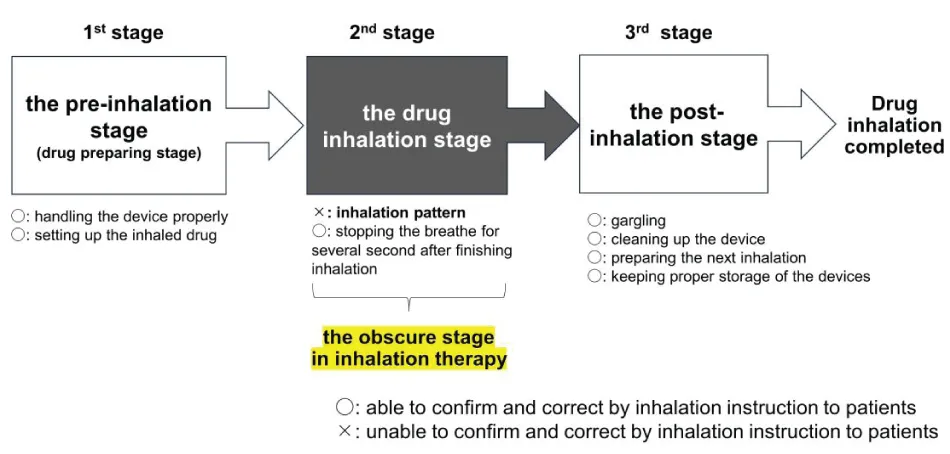
The three stages of the inhalation procedure in inhalation therapy
The sequence of the inhalation procedure in inhalation therapy can be divided into three successive stages: The first stage, the pre-inhalation stage; the second stage, the drug inhalation stage; and the third stage, the post-inhalation stage (Figure 1). In the pre-inhalation stage, patients prepare the inhaler for drug administration. Because each inhaler has a unique operation method, it is often difficult for patients to complete the operation procedure. There have been numerous reports of operating errors during the handling of these inhalers. Mishandling of the inhaler affects the patient’s medication adherence, satisfaction, and preferences [5]. In particular, critical errors in inhalation techniques often affect the health status and exacerbation in patients with asthma and COPD [6-8]. A previous clinical study assessed the most common errors in inhaler use over the past 40 years, in patients treated using pressurized Metered-Dose Inhalers (pMDI) or dry powder inhalers. According to this study, inhalation techniques have not improved over the past 40 years, suggesting an urgent need for education and new approaches to drug delivery [9]. Patient education should be repeated until patients can use the inhaler correctly. However, incorrect operation of the inhalation procedure varies from patient to patient. The cause of incorrect inhaler operation lies not only in the devices themselves but also in individual factors related to the background of the patient. Incorrect operation results from a confluence of factors such as age-related phenomena, physical disability, personal characteristics, personality, and lifestyle habits [3]. This makes inhalation therapy more complicated and cumbersome than traditional oral therapies. In the third stage, post-inhalation, patients undergo other laborious procedures. Patients are sometimes required to gargle and rinse off excess drugs from the oral cavity and throat, wash the device after inhalation, prepare for the next inhalation, and properly protect the inhalation device from excessive temperature changes and humidity. These processes are usually not necessary after administration of oral medicines. These problems, which occur during both the first and third stages, can often be solved by providing clear instructions to patients in clinical practice. However, the problems that arise in the second stage, the drug inhalation stage, cannot be solved by providing inhalation instructions to patients.
Serious problems in the second, drug inhalation, stage
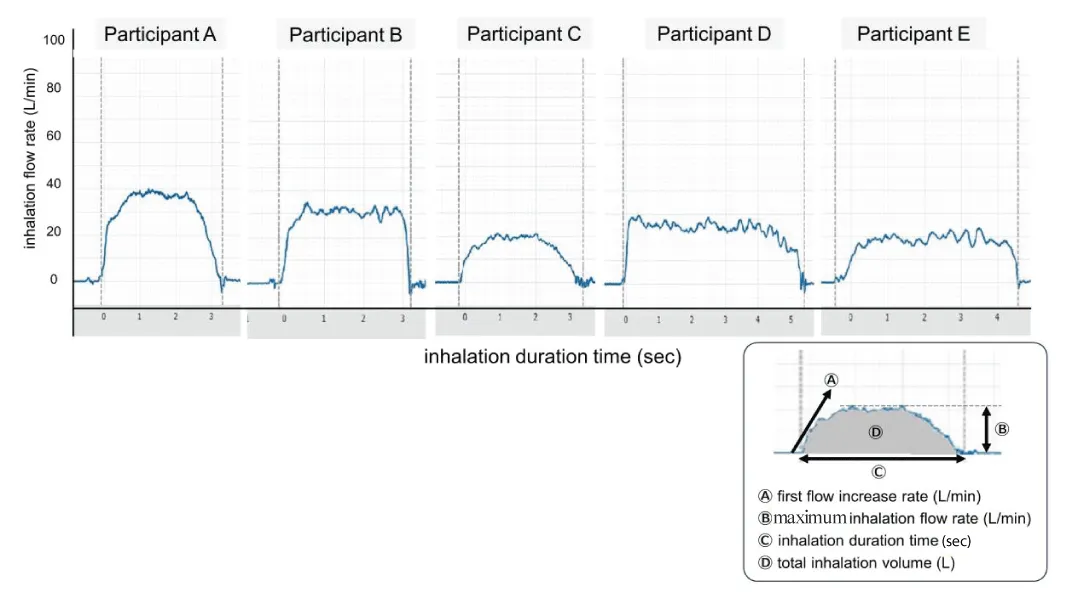
The second stage involves inhalation of the set drug. This is the most important stage of inhalation therapy. The total amount of drug delivered to the pulmonary area is determined by the patient's inhalation flow [10]. Therefore, the patient’s inhalation flow patterns play a pivotal role in the determinants of clinical performance of inhalation therapy [11,12]. Incorrect usage of inhaler devices has been a major serious problem, which spoils the benefits of inhalation therapy. In dry powder-type preparations, patients are generally taught to inhale the drugs both strongly and deeply. However, in aerosol-type preparations (pMDI), patients are generally required to synchronize their respiration with drug spraying and are instructed to inhale the drug deeply and slowly. On the other hand, the inhalation instructions are read by the patients themselves as a description of how to inhale or are often stated orally during patient education. Actual inhalation is usually performed with each patient individually interpreting and determining the optimal inhalation dose according to the description. Figure 2 shows the various patterns of individual inhalation, despite the same verbal instruction of inhalation. These results were produced by the measuring monitor, Tokico Inhalation MonitorTM (TIM) developed by Tokico System Solutions, Ltd. (Kanagawa, Japan). We noticed that the pattern of inhalation varies greatly, depending on each individual’s judgment and interpretation of the optimal inhalation method. Major indexes such as Ⓐ the first flow increase rate, Ⓑ inhalation flow rate, Ⓒinhalation duration, and Ⓓ total inhalation volume vary individually (Figure 2). This variation is caused by a lack of standardized inhalation patterns and is not necessarily due to physical differences among individual patients. The inhalation method considered suitable by the patient is not always optimal for the device. Neither the patient nor the instructor knows whether the drug is being inhaled appropriately or effectively. The most serious problem is the fact that this inhalation stage itself is “an obscure stage” in inhalation therapy, which is not well studied or understood. A method of measuring inhalation and determining its efficacy has not yet been developed. The inspiratory flow meter (InCheck® manufactured by Clement Clarke Int. Ltd., Essex, UK) is often used in clinical practice. This instrument is often used to confirm the patient’s potential to inhale at a maximum inspiratory airflow velocity that exceeds the minimum inspiratory speed required for each dry-powder-type inhaler. The parameter measured using this instrument is the peak intake velocity, which is fundamentally different from the measurement of inhalation patterns. A previous in vitro study showed that human inspiratory flow patterns reproduced by a mechanical simulator could be characterized by three parameters: inspiratory flow volume (Area under the Flow Rate-Time Curve (AUC), Flow Increase Rate (FIR) and Peak Flow Rate (PFR) [13]. This study showed that PFR, but not FIR or AUC, greatly affected the inhalation performance of physically mixed dry powders. However, unlike in vitro instrumental studies, in the case of actual patients, the measurement conditions are not constant; the inhalation pattern of individuals fluctuates greatly under various patient conditions, and it is not always possible to maintain the same inhalation pattern. Therefore, it is necessary to capture individual inhalation patterns in real-time for each patient. If we can capture the inhalation pattern of each patient and instruct them to adjust their pattern most appropriately according to the inhaler used, we believe that the therapeutic accuracy of inhalation therapy will be further improved. Nevertheless, few instruments have been developed to measure inhalation patterns. Recently, the importance of maintaining good medication adherence among patients has been discussed. To encourage patient adherence, whether the drug should be inhaled once or twice a day, and in accordance with patient preference, has been debated [14]. The content is very important, but the discussion may be premature until the “black box” of inhalation is resolved.
A new real-time inspiratory air velocity measuring monitor
Recent studies showed the efficacy of electronic medication monitoring in inhalation therapy [15]. Modernizing inhaled medications through digital technology can help address persistent problems of nonadherence and poor inhaler technique in patients with obstructive lung disease [16-18]. Self-reported inhalation using electronic medication monitors provided clinicians with accurate data on inhalation medication-taking behavior, thus reducing medication regimen complexity, side effects, and costs [19,20]. However, the mechanical approach against the obscure inhalation stage in inhalation therapy has been scarce.
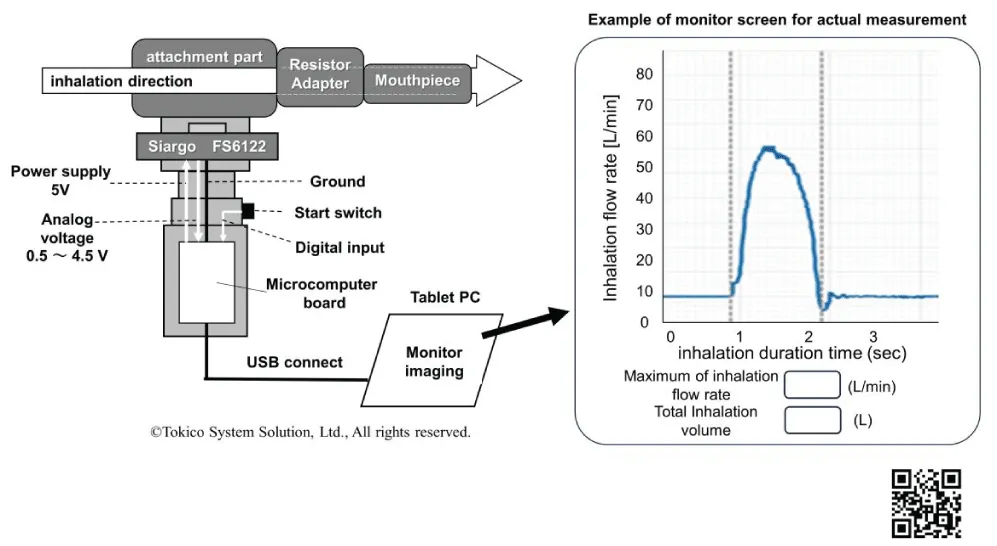
The TIM, originally developed by Tokico System Solutions, Ltd., is a measuring monitor that can draw inhalation patterns in real-time, and take an actual measurement of the inhalation flow rate and total inhalation volume [21]. Validation studies to determine measurement accuracy and reproducibility have already been completed [21]. In a collaboration study between Tokico System Solutions, Ltd. and the author, the TIM is currently being investigated for practical use. An outline of the TIM device configuration is shown in Figure 3. Figure 3 also shows an instructional video with the video URL [22]. When the power switch is pressed, the measurement mode is immediately initiated, and it can be measured, quickly, easily, and smoothly without invasiveness; therefore, it can be repeated many times in clinical practice.
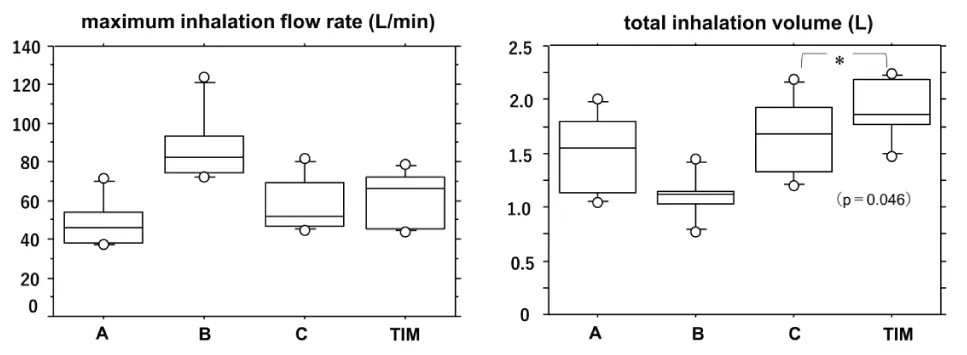
The TIM can measure inspiratory speed, inhalation duration, and total inhalation volume simultaneously and display them on the screen in real-time. Therefore, it is possible to provide guidance while correcting the inhalation pattern each time. Figure 4 shows the results of self-adjustment of the inhalation patterns using TIM in healthy participants. The total inhalation volume significantly improved when the participants checked their own inhalation patterns in real-time while watching the TIM screen, and when self-adjusting their inhalation flow rate and inhalation duration, they inhaled more effectively.
Conclusion
This device has entered the preliminary stage of development toward practical application for commercialization. In conclusion, we anticipate that the effective use of the TIM may shed light on the inside of the “black box of drug inhalation” and can be realized as a more effective method of inhalation therapy. Good inhalation enhances patient quality of life, facilitating improvements in adherence, treatment satisfaction, and motivation.
Conflict of interest
This research was a collaborative study with Tokico System Solutions, Ltd. To conduct this research, the company lent us a new real-time inspiratory air velocity measuring monitor (TIM) at no charge. There are no further matters to be reported regarding the implementation of this study.
Institutional review board statement
The study of the TIM in healthy participants strictly adhered to the ethical principles detailed in the Declaration of Helsinki (revised in 2013) and the “Ethical Guidelines for Human Life Science and Medical Research Guidance (established on April 16, 2021)” and was approved by the Ethical Review Committee (Clinical Research Tokyo Hospital Ethical Review Committee, first on July 24, 2014, and revised on March 24, 2022, approval number: 14072400).
Informed consent statement
Informed consent was obtained from all participants involved in the study. The participants were fully debriefed on the content of the research trial, and written consent was obtained. Written informed consent was also obtained from all of the participants for the publication of this paper.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (updated 2023) Chapter 3. Treating asthma to control symptoms and minimize risk. 48-138. https://ginaasthma.org/reports/
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 Report. Chapter 3: Evidence supporting prevention and maintenance therapy. 51-107. https://goldcopd.org/2023-gold-report-2/
- Ohbayashi H. Key points in effective inhaler technique training for asthma and COPD patients. Open J Pharmacol Pharmacother. 2020; 5: 27-29.
- Bosnic-Anticevich S, Bender BG, Shuler MT, Hess M, Kocks JWH. Recognizing and Tackling Inhaler Technique Decay in Asthma and Chronic Obstructive Pulmonary Disesase (COPD) Clinical Practice. J Allergy Clin Immunol Pract. 2023 Aug;11(8):2355-2364.e5. doi: 10.1016/j.jaip.2023.04.031. Epub 2023 May 3. PMID: 37146881.
- Ohbayashi H, Asano T, Kudo S, Ariga M. Comparison of User Satisfaction and Preference with Inhalant Devices Between a Pressurized Metered-Dose Inhaler and Ellipta in Stable Asthma Patients: A Randomized, Crossover Study. Pulm Ther. 2021 Jun;7(1):171-187. doi: 10.1007/s41030-021-00149-6. Epub 2021 Mar 2. PMID: 33651301; PMCID: PMC8137762.
- Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, Serra M, Scichilone N, Sestini P, Aliani M, Neri M; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011 Jun;105(6):930-8. doi: 10.1016/j.rmed.2011.01.005. Epub 2011 Mar 2. Erratum in: Respir Med. 2012 May;106(5):757. DelDonno, Mario [corrected to Del Donno, Mario]. PMID: 21367593.
- Usmani OS, Lavorini F, Marshall J, Dunlop WCN, Heron L, Farrington E, Dekhuijzen R. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018 Jan 16;19(1):10. doi: 10.1186/s12931-017-0710-y. PMID: 29338792; PMCID: PMC5771074.
- Kocks J, Bosnic-Anticevich S, van Cooten J, Correia de Sousa J, Cvetkovski B, Dekhuijzen R, Dijk L, Garcia Pardo M, Gardev A, Gawlik R, van der Ham I, Janse Y, Lavorini F, Maricoto T, Meijer J, Metz B, Price D, Roman Rodriguez M, Schuttel K, Stoker N, Tsiligianni I, Usmani O, Voorham J, Leving MT. Identifying critical inhalation technique errors in Dry Powder Inhaler use in patients with COPD based on the association with health status and exacerbations: findings from the multi-country cross-sectional observational PIFotal study. BMC Pulm Med. 2023 Aug 17;23(1):302. doi: 10.1186/s12890-023-02566-6. PMID: 37592263; PMCID: PMC10433653.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest. 2016 Aug;150(2):394-406. doi: 10.1016/j.chest.2016.03.041. Epub 2016 Apr 7. PMID: 27060726.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011 Mar 19;377(9770):1032-45. doi: 10.1016/S0140-6736(10)60926-9. Epub 2010 Oct 29. PMID: 21036392.
- Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000 Feb;117(2):542-50. doi: 10.1378/chest.117.2.542. PMID: 10669701.
- Bouwmeester C, Kraft J, Bungay KM. Optimizing inhaler use by pharmacist-provided education to community-dwelling elderly. Respir Med. 2015 Oct;109(10):1363-8. doi: 10.1016/j.rmed.2015.07.013. Epub 2015 Jul 20. PMID: 26341546.
- Hira D, Okuda T, Kito D, Ishizeki K, Okada T, Okamoto H. Inhalation performance of physically mixed dry powders evaluated with a simple simulator for human inspiratory flow patterns. Pharm Res. 2010 Oct;27(10):2131-40. doi: 10.1007/s11095-010-0215-6. Epub 2010 Jul 14. PMID: 20628789.
- van Boven JFM, Achterbosch M. Once- or Twice-Daily Inhaler Therapy for Optimal Adherence: "No-Brainer" or Shared Decision? J Allergy Clin Immunol Pract. 2023 Jul;11(7):2094-2095. doi: 10.1016/j.jaip.2023.05.006. PMID: 37422325.
- Pleasants RA, Chan AH, Mosnaim G, Costello RW, Dhand R, Schworer SA, Merchant R, Tilley SL. Integrating digital inhalers into clinical care of patients with asthma and chronic obstructive pulmonary disease. Respir Med. 2022 Dec;205:107038. doi: 10.1016/j.rmed.2022.107038. Epub 2022 Nov 7. PMID: 36446239.
- Stanford RH, Averell CM, Johnson PT, Buysman EK, Carlyle MH. Adherence and usage patterns of inhaled corticosteroids-long-acting beta-agonists by using inhaler-monitoring technology. Allergy Asthma Proc. 2020 Jul 1;41(4):256-264. doi: 10.2500/aap.2020.41.200037. PMID: 32605696.
- Chan AHY, Pleasants RA, Dhand R, Tilley SL, Schworer SA, Costello RW, Merchant R. Digital Inhalers for Asthma or Chronic Obstructive Pulmonary Disease: A Scientific Perspective. Pulm Ther. 2021 Dec;7(2):345-376. doi: 10.1007/s41030-021-00167-4. Epub 2021 Aug 11. PMID: 34379316; PMCID: PMC8589868.
- Tay TR, van Boven JFM, Chan A, Hew M. Electronic Inhaler Monitoring for Chronic Airway Disease: Development and Application of a Multidimensional Efficacy Framework. J Allergy Clin Immunol Pract. 2022 May;10(5):1189-1201.e1. doi: 10.1016/j.jaip.2021.11.027. Epub 2021 Dec 13. PMID: 34915225.
- Mosnaim GS, Stempel DA, Gonzalez C, Adams B, BenIsrael-Olive N, Gondalia R, Kaye L, Shalowitz M, Szefler S. Electronic medication monitoring versus self-reported use of inhaled corticosteroids and short-acting beta2-agonists in uncontrolled asthma. J Asthma. 2022 Oct;59(10):2024-2027. doi: 10.1080/02770903.2021.1996600. Epub 2021 Nov 2. PMID: 34699302.
- van de Hei SJ, Kim CH, Honkoop PJ, Sont JK, Schermer TRJ, MacHale E, Costello RW, Kocks JWH, Postma MJ, van Boven JFM. Long-Term Cost-Effectiveness of Digital Inhaler Adherence Technologies in Difficult-to-Treat Asthma. J Allergy Clin Immunol Pract. 2023 Jul 3:S2213-2198(23)00716-X. doi: 10.1016/j.jaip.2023.06.051. Epub ahead of print. PMID: 37406806.
- Ohbayashi H, Horikoshi S, Ishizeki K. Efficacy of inhalation instruction using a new real-time inspiratory air velocity measuring monitor. Insights of Clinical and Medical Images. 2023; 5: 1-10.
- Tokico Inhalation Monitor TM. 2023. https://www.youtube.com/watch?v=KSDQMe_BdW8
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
