International Journal of Pharmaceutical Sciences and Developmental Research
Physicochemical characterization and biological activity of polysaccharides from the seeds of the turnip Brassica rapa
Yuliya I Oshchepkova*, Мunojat J Oripova, Zulfizar N Kuzieva, Barno B Koraboeva, Dilnoza G Abdugafurova, Dilfuza A Amanlikova and Shavkat I Salikhov
Cite this as
Oshchepkova YI, Oripova МJ, Kuzieva ZN, Koraboeva BB, Abdugafurova DG, et al. (2023) Physicochemical characterization and biological activity of polysaccharides from the seeds of the turnip Brassica rapa. Int J Pharm Sci Dev Res 9(1): 019-027. DOI: 10.17352/ijpsdr.000048Copyright License
© 2023 Oshchepkova YI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Analysis of the monosaccharide composition showed that the composition of the neutral polysaccharide BSP-1-1 is represented by monosaccharides in the following composition: ribose – 5.05%, arabinose – 56.38%, mannose – 5.87%, glucose – 8.63% and galactose – 24.05%. The composition of the anionic polysaccharide BSP-2-1 is represented by monosaccharides: ribose – 6.35%, arabinose – 60.15%, mannose – 7.19%, glucose – 4.12% and galactose – 22.16%. It was determined that the isolated polysaccharides consist mainly of arabinose (BSP-1-1 – 56.3%, BSP-2-1 – 60%) and galactose (BSP-1-1 – 24%, BSP-2-1 – 22%). Based on the data obtained, it can be assumed that the studied polysaccharides from the seeds of the turnip Brassica rapa belong to the type of arabinogalactans. According to the results of studying the properties of acute toxicity of the BSP polysaccharide from the seeds of the turnip Brassica rapa, it was found that this substance belongs to class V compounds - practically non-toxic substances. With intragastric administration of inulin at doses of 25, 50, and 75 mg/kg and BSP polysaccharide from the seeds of the turnip Brassica rapa at doses of 10, 20 and 30 mg/kg 14 days after the induction of diabetes mellitus, the indicator was close to that of the intact group of animals (intact - 3.07 ± 0.25, inulin 2.99 - 3.14 ± 0.23, and BSP polysaccharide from the seeds of the turnip Brassica rapa 2.53-3.14 ± 0.20), while the indicator of the control group was - 8.40 ± 0.35.
This study will provide an opportunity to plan and conduct studies to study the action of substances in the metabolism of glucose and lipids, which examines the enzymes that regulate the lipid activity of the liver, as well as the morphology of the liver and adipose tissue.
Introduction
Currently, diabetes mellitus is one of the most common diseases that are gaining increasing social importance. If 15 years ago, WHO assumed that by 2025 the number of patients with diabetes in the world would be 380 million people [1], then according to the International Diabetes Association (IDF), in 2021 the number of patients with diabetes has already exceeded 536 million and according to its forecasts by 2045, this number will increase by 46%, reaching 783.2 million people [2]. Diabetes mellitus is a chronic disease characterized by high levels of glucose in the blood, leading to systemic disorders of all types of metabolism and damage to the micro- and macrovascular systems of the body [3]. Most researchers identify the following factors contributing to the development of type 2 diabetes: dietary disorders associated with the consumption of high-calorie, fatty foods high in easily digestible carbohydrates, and a sedentary lifestyle. According to modern research, prolonged consumption of fatty foods with a high content of carbohydrates leads to a violation of the compositional composition of the intestinal microflora, which leads to the development of "low-grade inflammation". As shown by a number of researchers, this ultimately leads to the development of insulin resistance in insulin-dependent tissues (muscles, liver, and adipose tissue), i.e., to a systemic decrease in sensitivity to the anabolic hormone insulin [4,5].
Polysaccharides are the most common natural macromolecular polymers that are obtained from renewable sources such as algae, plants, and microorganisms such as fungi and bacteria [6]. Together with other biomolecules such as proteins and nucleotides, polysaccharides are an important component and perform many functions in the biological system, such as intercellular communication, adhesion, and molecular recognition in the immune system [7]. Polysaccharides, which belong to the third major class of biopolymers (carbohydrates), play a critical role in many different physiological processes and tumor metastasis [8], they can provide structure, protection, adhesion, and response to stimuli, and also play a critical role in the immune system, blood coagulation, fertilization, prevention of pathogenesis and therapeutic efficacy [9].
Phyto preparations based on polysaccharides are used as an expectorant and anti-inflammatory drugs [10], their enveloping and emollient properties, anti-hypoxic, antioxidant, hepatoprotective and radioprotective effects are widely known [10,11]. A number of researchers emphasize the prospects of using polysaccharides for the correction of lipid metabolism and in the treatment of diabetes mellitus [12,13].
Vegetables of the Brassicaceae family are considered part of the human diet, and consumed by the population of the whole world. Many studies have shown that there is an inverse relationship between the consumption of Brassicaceae vegetables and the risk of chronic diseases, especially such as cardiovascular disease, cancer, Alzheimer's disease, cataracts, and age-related functional decline [14,15].
The turnip Brassica rapa L. is one of the oldest cultivated vegetables and is widely consumed in the highlands of Asian countries. For example, turnip root is eaten by Tibetan people living in the Qinghai-Tibet Plateau to relieve fatigue and prevent hypoxia [16]. Several studies of its chemical composition have shown that this plant is a rich source of glucosinolates [17], phenolic compounds, organic acids [18], flavonoids [19], sulfur compounds, and volatiles [20], which have been found to have various beneficial health effects. Several studies have shown turnips to exhibit biological activities, including antimicrobial and anticancer [21], antioxidant [17,22], and antidiabetic [23]. More recently, several new biological effects have been reported, such as the prevention of cerebral hypoxia and pulmonary edema [24,25], as well as the prevention of osteoporosis [26], which enrich the turnip as a functional food.
The purpose of this research is to isolate and study the physicochemical and biological properties of water-soluble polysaccharides in turnip seeds cultivated in Uzbekistan.
Methods
Plant material
Turnip seeds Brassica rapa L. were collected in July 2020 on the territory of the Republic of Uzbekistan (Namangan region, Mingbulak district).
Experimental chemical part
Degreasing and removal of low molecular weight impurities: For defatting, pre-crushed seeds were extracted with petroleum ether in a Soxhlet apparatus for 72 hours. Degreased seeds were dried at room temperature in the air. To remove low-molecular impurities and coloring substances, the raw material was extracted in a Soxhlet apparatus with a mixture of chloroform and ethyl alcohol 96% (1:2). The raw material was dried in air to remove the smell of solvents.
Extraction of water-soluble polysaccharides
To isolate water-soluble polysaccharides, defatted seeds were extracted three times with water in a water bath at 95 °C under reflux (the ratio of raw material and extractant was 1:20, 1:15, 1:15). The duration of each extraction was 2 h. The resulting aqueous extracts were combined and evaporated on a rotary evaporator at a temperature of 50 °C to 1/5 of the volume. From the resulting concentrate, water-soluble polysaccharides were precipitated by adding a fourfold volume of 96% ethanol and left at 4 °C overnight. The precipitate was separated by centrifugation, washed with ethanol, and freeze-dried.
Deproteinization of polysaccharides
Deproteinization of the sum of polysaccharides was performed according to the Savage method [27]. A 3-fold volume of CHCl3–n-BuOH (4:1 ratio) was added to an aqueous solution of the sum of polysaccharides and transferred to a separating funnel. The funnel was shaken vigorously for 5 min and the mixture was kept for 3 hours to achieve equilibrium of the two phases. The organic phase with residual proteins (lower layer) was removed. This procedure was repeated 6 times. Turnip seed polysaccharides were precipitated with three volumes of ethanol from the aqueous phase. After filtration, the precipitate was washed with absolute ethanol and dried in air.
Determination of the content of polysaccharides
The quantitative content of polysaccharides was determined by the phenol-sulfuric acid method [28] according to the calibration graph for glucose. Optical density was measured on a Metash UV-5100 spectrophotometer (Shanghai, China).
Ion exchange chromatography
Anion exchange chromatography was used to isolate the polysaccharide. 100 mg of a polysaccharide sample was dissolved in 5 ml of distilled water and applied to a column (16×3.5 cm) with DEAE-650C TOYOPEARL (TOSOH, Japan) equilibrated with distilled water. After loading the sample, the column was eluted with distilled water and then successively with a 0–1.0 M NaCl gradient solution at a rate of 1.0 mL/min. Fractions with a volume of 10.0 ml were collected with a fraction collector. The content of carbohydrates in the fractions was determined by the phenol-sulfuric acid method, using glucose as a standard. The fraction corresponding to the individual peaks was pooled, concentrated, dialyzed, and freeze-dried.
Gel filtration of polysaccharides
Neutral and eluted at 0.1 M NaCl polysaccharides (20 mg each) were dissolved in 2 ml of water and applied to a column (70×1.8 cm) with Sephadex G-100. The column was eluted with distilled water at a flow rate of 40 ml/h. The content of carbohydrates in the samples was determined by the phenol-sulfuric acid method, using glucose as a standard. Fractions with a volume of 13 ml were taken. Fractions corresponding to individual peaks were pooled, concentrated to the minimum volume, dialyzed, and freeze-dried.
Monosaccharide composition of polysaccharides
The polysaccharide after gel filtration (3 mg) was dissolved in 2.5 ml of 2 M trifluoroacetic acid in a 5 ml ampoule, hydrolyzed at 110 °С for 6 h, and the cooled reaction mixture was centrifuged at 3000 rpm for 5 min. To remove trifluoroacetic acid from the hydrolyzate, 5 ml of dry methanol solution was added three times, and the methanol was evaporated on a rotary evaporator. 5 mg of hydroxylamine hydrochloride and 1 mg of inositol were added to the dry hydrolyzate and dissolved in 0.5 ml of pyridine. The solution was heated at 90 °C for 30 min, cooled to room temperature, 0.5 ml of acetic anhydride was added, and acetylated for 30 min at 90 °C. The reaction mixture was dried under nitrogen flow. The alditol acetate derivatives of the monosaccharide standards (L-Fruc, L-Rib, L-Rha, L-Ara, L-Xyl, D-Man, D-Glc, and D-Gal) were prepared as described above. The synthesized alditol acetate derivatives were analyzed by gas chromatography/mass spectrometry GC/MS (column Thermo Finnigan TRACE 2000/MS, DB-5MS (30 m×0.25 mm×0.25 mm), temperature program from 180 to 270 °C at 20 °C C/min, holding 270 °C for 25 min). Peaks corresponding to alditol acetates and their fragments were determined by their mass spectra and GC separation times. The ratio of monosaccharides to polysaccharides was determined by comparing peak areas.
IR spectroscopy
The IR spectra of the samples were recorded on an IRTracer-100 SHIMADZU IR-Fourier spectrometer (Japan), system 2000 in the frequency range 400–4000 cm-1. The spectra of the studied samples were recorded by the method of Attenuated Total Internal Reflection (ATR) spectroscopy in the infrared region with Fourier transform spectroscopy.
Experimental biological part
The studies were carried out based on international and national regulatory documents [29-31] by qualified staff. Manipulation of laboratory animals was performed according to the manual of the European Convention for the protection of vertebrate animals used for experiments or other scientific purposes [32]. The protocol was approved by the Animal Ethical Committee based on the Institute of Bioorganic Chemistry, AS RUz (Protocol Number: 133/1a/h, dated August 4, 2014).
In the experiments, we used outbred rats (150–200 g) and mice (23–25 g) grown in vivarium conditions of the Laboratory of Pharmacology and Screening of Biologically Active Compounds of the Institute of Bioorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan in standard access to food and water. The experiments were carried out on healthy rats and mice that were kept in a vivarium and passed a quarantine period of at least 10-14 days.
Acute toxicity study
In experiments to study the acute toxicity of the BSP polysaccharide from the seeds of the turnip Brassica rapa, it was determined in white mice with a single oral administration by the Lichfield and Wilcoxon method. The experimental groups consisted of 5 animals of both sexes. The studied polysaccharide BSP from the seeds of the turnip Brassica rapa was injected into the stomach of mice through a special probe at doses of 2000–2001 mg/kg. On the first day of the experiment in laboratory conditions, the general condition of the animals of the experimental and control groups was checked hourly. In vivarium conditions, daily checks of general condition, activity, coat, skin and tail condition, behavior, frequency and depth of respiration, amount and consistency of feces (soft or hard), frequency of urination, changes in body weight, and other indicators. During the experiment, the animals were fed in the usual way, in conditions where water and food were not limited. At the end of the experiment, the average lethal dose (LD50) of the test sample and, accordingly, the toxicity class were determined.
Study of the effect on immunity
The study of the effect of polysaccharides on animal immunity was carried out on model reactions for assessing antibody formation in mice when immunized with antigens (humoral immune response) and inducing a delayed-type hypersensitivity reaction (DTH) to an antigen (cellular immune response).
Study of the effect on humoral immunity
To study the effect of BSP on humoral immunity, the number of Antibody-Forming Cells (AFC) in the spleens of mice immunized with ram erythrocytes (EB) was determined by the method of Jerne, Nordin.
As a result of sowing cells of the spleen of mice immunized with antigens (EB) using complement-guinea pig serum, on an opaque background of agarose, plaques of local hemolysis were clearly visible - transparent zones about 1 mm in diameter, which are antibody-forming cells with sheep erythrocytes lysed around. The number of cells producing IgM antibodies was determined by the number of plaques on the dish. 0.05 ml of spleen cell suspension, that is, 1/200 of the spleen suspension in 10 ml of Hank's solution, was inoculated per dish. To determine the absolute number of AFCs accumulating in the spleen of mice, the number of AFCs calculated over the entire dish was multiplied by 200. To recalculate the number of AFCs per 1 million nucleated spleen cells, the number of millions of cells per entire spleen was counted in the Goryaev chamber. By dividing the absolute number of AFCs by the number of millions of cells, the number of AFCs per 1 million nucleated spleen cells was determined. On the day of immunization, mice of the experimental groups were intragastrically injected with an aqueous solution of the drug at a dose of 5, 0.5, and 0.05 mg/kg in a volume of 0.2 ml. The control group of mice received water in the same volume.
Study of the effect on cellular immunity
To study the effect of BSP on cellular immunity, a delayed-type hypersensitivity reaction (DHRT) was used. When staging the reaction of HCHST, the antigen is administered twice for sensitization for resolution. Sensitization with protein antigens causes the formation of antigen-specific T-lymphocytes, which, upon repeated administration of the antigen, specifically interact with it and release a number of pro-inflammatory cytokines.
The experiments used white outbred mice weighing 18-24 grams. For the development of the HPRT reaction, mice were subcutaneously immunized with 1x10 ram erythrocytes in 0.2 ml of saline solution. On the 5th day, mice were injected with a resolving dose of 1x108 EB in 0.05 ml of saline into the pad of the hind paws, and the same volume of saline was injected into the contralateral paw as a control. After 24 hours, the local inflammatory reaction was assessed by the difference in the mass of the experimental and control paws, and the reaction index was calculated using the formula:
Where Ро – the mass of experienced paw,
Рк – control paw weight.
On the day of immunization, mice were injected intravenously in a volume of 0.1 ml/mouse with an aqueous solution of drugs - 5 mg/kg. The control group of mice received intragastrically distilled water in the same volume [30]. In studies, a pharmaceutical preparation of plant origin - Immunal tablets (Sandoz, d.d., Slovenia) at a dose of 60 mg/kg was used as a reference drug. The calculation of the administered dose was carried out according to the formula:
Modeling chemically induced diabetes mellitus
In the pathogenesis of type 1 diabetes mellitus, external and internal environmental factors (viruses, chemicals, nutritional factors, interleukin 1β) cause the activation of the free radical oxidation process in cells. Under the influence of free radicals, β-cell proteins change their natural properties (denature) and become antigens for their own immune system. The increased sensitivity of pancreatic β-cells to the action of free radicals is explained by the fact that they have reduced activity of their own antioxidant defense system. Free radicals cause disturbances in the DNA structure of insulin-producing cells, which leads to the subsequent death of β-cells. The most common models of type 1 diabetes are chemically induced diabetes mellitus and surgical diabetes mellitus.
To study the hypoglycemic activity of the polysaccharide BRP from the seeds of the turnip Brassica rapa, a model of chemically induced diabetes mellitus using alloxan was used.
To induce type 1 diabetes mellitus in rats, alloxan (Sigma-Aldrich, USA) was injected intraperitoneally at a dose of 150 mg/kg. Alloxan was administered to animals after 36 h of fasting, which provides better reproducibility of these models due to a decrease in the level of glycemia to the lower limit of the reference range and a decrease in the variability of its level between animals [33].
Alloxan (2,4,5,6 - tetraoxohexahydropyrimidine) is a uric acid derivative that has a cytotoxic effect on pancreatic β-cells. Alloxan increases the level of Reactive Oxygen Species (ROS) and promotes selective necrosis of pancreatic β-cells. When administered parenterally, this substance exhibits a diabetic effect. Alloxan contributes to the development of persistent DM through damage to β-cells of the pancreatic islets. This compound has minimal effect on other tissues when administered at the proper dose and is selective for islet β cells. Induction of DM by alloxan is associated with partial destruction of pancreatic islet cells, which leads to a decrease in the quantity and quality of insulin. Alloxan selectively inhibits insulin secretion by blocking the activity of glucokinase, the β-cell glucose sensor. Alloxan induces insulin-dependent diabetes by inducing the formation of ROS, which leads to necrosis of β-cells. It enters β-cells, participates in a number of processes that can damage β-cells and cause necrosis, and also generates ROS in a cyclic reaction with dialuric acid, its reduction product. The toxicity of alloxan is enhanced by ROS generated during this redox reaction [34].
Study design
On the second day after the induction of diabetes mellitus, the animals were intragastrically injected with BSP polysaccharide from the seeds of turnip Brassica rapa at doses of 10, 20 and 30 mg/kg and inulin (Now Foods dietary supplement (USA). Inulin Сertified Organic. Prebiotic Pure Powder - as a reference substance as antidiabetic agent for the prevention and treatment of diabetes mellitus) at doses of 25, 50 and 75 mg/kg for 14 days. On the 7th and 14th days, the animals' blood was taken to determine the glucose content and hematological analysis.
Glucose content was determined using blood serum on an FEK instrument at a wavelength of 540 nm using KIT Cypress Diagnostics (Belgium).
Peripheral blood parameters: hemoglobin content, erythrocyte count, average erythrocyte volume (MCV), average hemoglobin content in an individual erythrocyte (MCH), average hemoglobin concentration in erythrocyte mass (MCHC), number of reticulocytes, platelets, leukocytes, concentration of lymphocytes (Lym, %), erythrocytes (RBC, 1012/l), platelets (PLT, 109/l) were measured on an automatic hematology analyzer Dymind DH36, Shenzhen Dymind Biotechnology Co., ltd, China.
Statistical processing of results
Statistical analysis of the results of the study was carried out on the basis of determining the arithmetic means (M) and their errors ( ± m) using standard programs of the Microsoft Office package.
Results
Isolation and purification of polysaccharides
Extraction with water was used to isolate the polysaccharides. Polysaccharides (conventionally named BSP) were precipitated from aqueous solutions with the addition of ethanol in a ratio of 1:4 (by volume). The yield of polysaccharides was 1.6%. The total amount of carbohydrates was 30.3%, which indicates the presence of impurities in the composition of the isolated polysaccharide. The isolated polysaccharides were then deproteinized by the Savage method. After deproteinization, the amount of protein in the samples was determined by the Lowry method. The results showed that the isolated polysaccharide contained trace amounts of proteins and peptides.
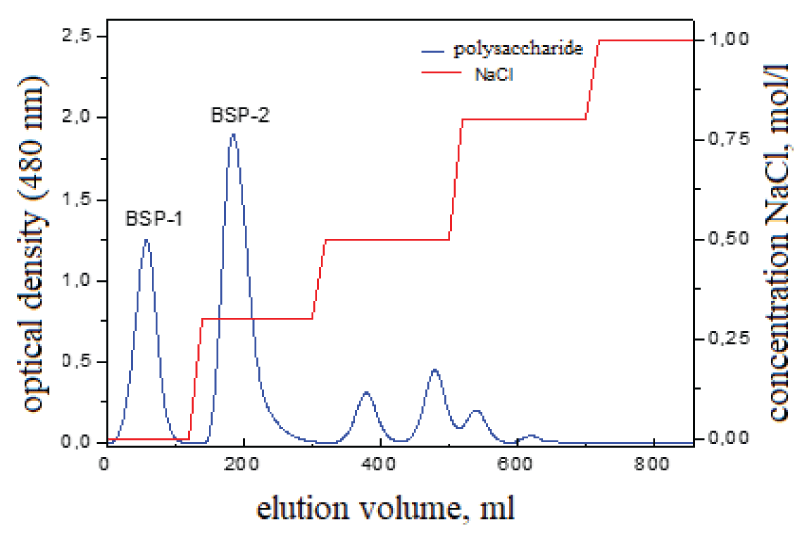
The polysaccharide sample was passed through DEAE-650C, washed with water, and eluted with a subsequent NaCl gradient solution (0–1 M). As shown in Figure 1, neutral polysaccharides were obtained by washing with distilled water to obtain one polysaccharide fraction (conventionally named BSP-1), and anionic polysaccharides were eluted with 0.1 M NaCl solution (conventionally named BSP-2). This indicates that this sample is composed of a neutral and anionic polysaccharide.
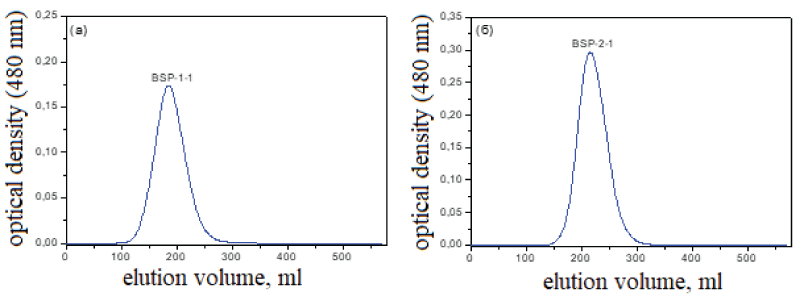
The major polysaccharide fractions of BSP-1 and BSP-2 were further separated and sequentially purified on a Sephadex G-100 column (Figure 2) and it was determined that the polysaccharide samples consisted of homogeneous polysaccharides. The two major polysaccharide peaks, BSP-1-1 and BSP-2-1, were collected and freeze-dried.
Рhysico-chemical characteristics of polysaccharides
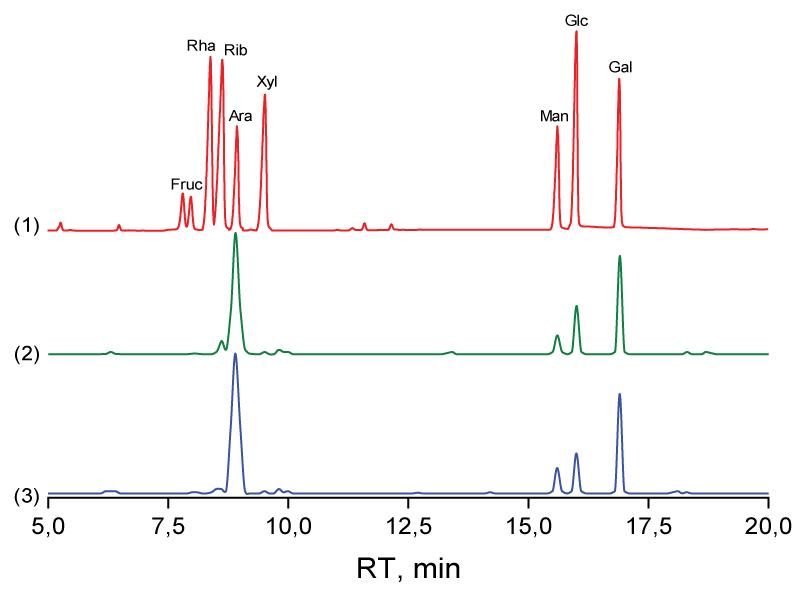
The monosaccharide composition of BSP-1-1 and BSP-2-1 was determined by hydrolysis of trifluoroacetic acid and the GC/MS method (Figure 3). The results obtained are presented in Table 1. From the results obtained, it can be seen that the polysaccharides consist mainly of arabinose and galactose residues. Other monosaccharides are present in their composition in trace amounts. Comparison with the monosaccharide composition of previously isolated polysaccharides from turnip roots showed that the roots contain polysaccharides of a different composition containing arabinose and glucose in BRP-1-1 in a molar ratio of 1.66:98.34; in BRP-2-1, arabinose, galactose, and glucose in a molar ratio of 9.3:14.63:76.07; and in BRP-3-1, arabinose, rhamnose, galactose, and glucose in a molar ratio of 24.98:24.10: 44.09:6.83 [35].
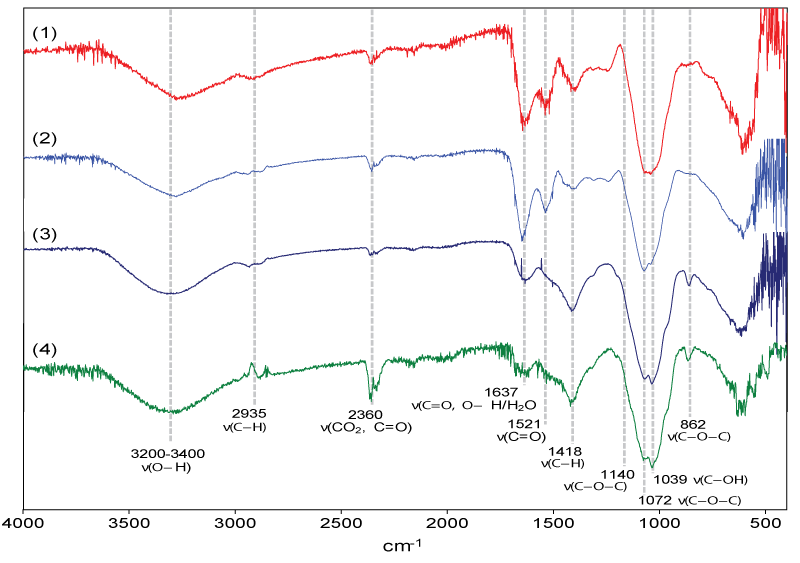
The isolated polysaccharides were subjected to IR spectroscopic studies (Figure 4). Absorption bands corresponding to polysaccharides were observed in the IR spectrum. The band between 3200–3400 cm-1 represents the О-Н stretching vibrations. Characteristic signals for symmetric stretching of H–C–H bonds were observed at 2935 cm–1. In the region of 2360 cm-1, characteristic signals were observed corresponding to the C=O bond of adsorbed CO2. In the region of 1637 cm–1, absorption bands were observed that are characteristic of the O–H bound water molecule in the samples and protein C=O bonds. Asymmetric stretching vibrations at 1521 cm-1 corresponding to C=O (specific for proteins and peptides) were observed only in the crude and deproteinized extract. The deproteinized extract had a much-reduced peak intensity. This shows that after deproteinization, the number of proteins and peptides decreased significantly. No peaks were observed in this region for BSP-1-1 and BSP-2-1, which indicates that the samples were completely free of proteins and peptides after separation.
Absorption at 1418 cm-1 is an asymmetric stretching vibration of the C-H bond (CH2 groups) corresponding to polysaccharides. The characteristic peaks for the C-O-C bond in the pyranose ring of the monosaccharide unit of the polysaccharides were observed at 1140 cm-1. Absorption bands of stretching vibrations corresponding to glycosidic С-О-С bonds between monosaccharide residues were observed in the region of 1072 cm-1. Absorption at 1039 cm-1 represents C-O stretching vibrations from side carbinol groups (C-OH). Characteristic signals of deformation vibrations of α-glycosidic bonds between pyranose forms of polysaccharides were found at 862 cm-1.
IR spectroscopic studies have shown that the isolated polysaccharides from the seeds of the turnip Brassica rapa consist mainly of α-linked pyranose residues.
Polysaccharides consisting of xylose, mannose, and galactose are believed to reduce hyperlipidemia in rat models, and therefore the biological activity was studied in a model of diabetes mellitus.
Toxicity and immunomodulatory studies in animals
After 10-15 minutes, with oral administration of BSP polysaccharide at a dose of 2000 mg/kg, tachycardia, an increase in the number of washings, and grouping of animals were observed, which disappeared after 20 minutes. When observing animals for 14 days, no deaths were recorded. It was determined that with a single oral administration of the BSP polysaccharide from the seeds of the turnip Brassica rapa to animals at doses of 2000 and 2001 mg/kg, no animal deaths were recorded during the 14 days of the experiment.
According to the results of the study of the acute toxicity properties of the BSP polysaccharide from the seeds of the turnip Brassica rapa, it was found that this substance belongs to class V compounds - practically non-toxic substances.
Based on the results of acute toxicity studies, doses were selected for further studies.
Evaluation of the effect of BSP on humoral immunity
The reference drug was Immunal® tablets (SANDOZ, d.d., Slovenia), an herbal medicinal product containing juice from freshly harvested flowering parts of the Echinacea purpurea plant. Echinacea purpurea contains biologically active substances that stimulate the nonspecific immune system and strengthen the body's defenses. The main active ingredients of Echinacea purpurea are caffeic acid derivatives (chicory acid and its esters), alkamides, and polysaccharides, which exhibit immunostimulatory activity.
The results of the experiments showed that BSP in the studied doses, compared with the pharmaceutical herbal preparation Immunal, has a pronounced stimulating effect on the process of antibody production in mice. A significant increase in antibody production was found after intragastric administration of BSP at doses of 0.05, 0.5, and 5 mg/kg, which proves the stimulating effect of the drug at doses of 0.05 and 0.5 mg/kg (S1 Table). Thus, the results of the experiments showed that BSP at the studied doses has a pronounced stimulating effect on the process of antibody formation in mice, that is, on the humoral immunity of animals.
Evaluation of the effect of BSP on cellular immunity
The conducted studies showed that under the influence of BSP in all studied doses (0.05; 0.5 and 5 mg/kg) in relation to the reference drug Immunal at a dose of 60 mg/kg, a dose-dependent decrease was observed (decrease in the difference in masses of the experimental and control paws) index of response to inflammation and there was a significant trend towards a decrease in the development of delayed-type hypersensitivity reactions, especially at doses of 0.5 and 5 mg/kg (S2 Table).
As a result of the experiments on the effect of BSP on humoral and cellular immunity, it was found that after a single injection of the test substance simultaneously with the antigen (RE) at doses of 0.05 and 0.5 mg/kg, a significant increase in antibody production was found in mice and no significant increase was noted. Delayed-type hypersensitivity reactions in mice at all studied doses in relation to the control.
When studying the immunomodulatory properties of BSP, it was shown that the substance does not cause changes in immunity, which are detected by the effect on the lymphoid organs.
Evaluation of the effect of BSP on models of alloxan diabetes
The introduction of alloxan causes a pronounced toxic effect on β-cells of the pancreas: 7 days after the administration in animals of the control group, the concentration of glucose in the blood was maximum and amounted to 16.2 ± 0.12 mmol/l. This indicates a pronounced violation of carbohydrate metabolism due to the destruction of pancreatic β-cells.
In animals after the introduction of polysaccharide BSP from the seeds of the turnip Brassica rapa and inulin in small doses, the content of glucose in the blood of animals during the measurement was significantly lower than in the control group. Glucose concentration in groups of animals after taking BSP polysaccharide decreased by 35%, amounting to 10.5 ± 0.96 mmol/l, and after inulin - only by 24%, amounting to 12.3 ± 0.35 mmol/l. A week later, i.e. on the 14th day, the concentration of glucose in the blood of the test animals was close to the concentration of glucose in healthy animals - 3.07 ± 0.25 mmol/l.
Table 2 shows the indicators of blood glucose in the alloxan model rats on days 7 and 14 after the introduction of the BRP polysaccharide from the seeds of the turnip Brassica rapa at doses of 10, 20, and 30 mg/kg and inulin at doses of 25, 50 and 75 mg/kg.
Peripheral blood parameters were also measured after the application of BSP polysaccharide from turnip seeds Brassica rapa and inulin (S3, S4 Tables).
According to the results obtained, the data on the content of blood leukocytes in animals of intact and experimental groups in all studied doses of two substances on the 7th day differ sharply, which is consistent with the literature data, however, in large doses: 75 mg/kg of inulin and 30 mg/kg of polysaccharide BSP leukocytes are almost equal to that of the intact group.
The content of erythrocytes in all studied doses of both substances of the experimental groups does not differ from that of the intact group.
Within 14 days of treatment after the induction of diabetes mellitus, all blood counts and immunocompetent cells (leukocytes, erythrocytes, and platelets) with intragastric administration of inulin and the polysaccharide BSP from the seeds of the turnip Brassica rapa recovered to a normal physiological state, which indicates a potential antidiabetic and hypoglycemic effect studied substances.
Conclusion
For the first time, water-soluble polysaccharides were isolated from turnip seeds by sequential water extraction. The results of IR spectroscopic studies showed that the isolated polysaccharides consist mainly of α-linked pyranose units. It was determined that the isolated polysaccharides consist mainly of arabinose (BSP-1-1 – 56.3%, BSP-2-1 – 60%) and galactose (BSP-1-1 – 24%, BSP-2-1 – 22%). Based on the data obtained, it can be assumed that the studied polysaccharides from the seeds of the turnip Brassica rapa belong to the type of arabinogalactans.
When studying the immunomodulatory properties, it was determined that the polysaccharide BSP polysaccharide from the seeds of the turnip Brassica rapa does not cause changes in immunity, which are detected by the effect on the lymphoid organs, having a stimulating effect on the humoral and cellular immune response.
According to the results of studying the properties of acute toxicity of the BSP polysaccharide from the seeds of the turnip Brassica rapa, it was found that this substance belongs to class V compounds - practically non-toxic substances. With intragastric administration of inulin at doses of 25, 50, and 75 mg/kg and BSP polysaccharide from the seeds of the turnip Brassica rapa at doses of 10, 20, and 30 mg/kg 14 days after the induction of diabetes mellitus, the indicator was close to that of the intact group of animals (intact - 3.07 ± 0.25, inulin 2.99 - 3.14 ± 0.23, and BSP polysaccharide from the seeds of the turnip Brassica rapa 2.53-3.14 ± 0.20), while the indicator of the control group was - 8 .40 ± 0.35.
Within 14 days of treatment after the induction of diabetes mellitus, all blood counts and immunocompetent cells (leukocytes, erythrocytes, and platelets) with intragastric administration of inulin and BSP polysaccharide from the seeds of the turnip Brassica rapa recovered to a normal physiological state, which indicates a potential antidiabetic and hypoglycemic effect studied substances.
This study will provide an opportunity to plan and conduct studies to study the action of substances in the metabolism of glucose and lipids, which examines the enzymes that regulate the lipid activity of the liver, as well as the morphology of the liver and adipose tissue.
Credit authorship contribution statement
Yu.I. Oshchepkova: Conceptualization, Methodology
М.J. Oripova, Z.N. Kuzieva, B.B. Koraboeva, D.G. Abdugafurova, D.A. Amanlikova: Visualization, Investigation.
Sh.I. Salikhov: Supervision.
D.G. Abdugafurova, D.A. Amanlikova: Software, Validation.
Yu.I. Oshchepkova: Writing- Reviewing & Editing
- World Health Organisation. The World Health Report 1998. Life in 21st Century – a Vision for ALL. Geneva: World Health Organisation. 1998.
- Kurkin DV, Bakulin DA, Morkovin EI, Gorbunova YuV, Strygin AV, Andriashvili TM, Sokolova AA, Bolokhov NS, Pustynnikov VE, Fomichev EA. Hypoglycemic effect of sitagliptin and aminoguanidine combination in experimental diabetes mellitus. Pharmacy & Pharmacology. 2022;10(6):536-548. DOI: 10.19163/2307-9266-2022-10-6-536-548
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014 Jan;37 Suppl 1:S81-90. doi: 10.2337/dc14-S081. PMID: 24357215.
- Friedman M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods. 2016 Nov 29;5(4):80. doi: 10.3390/foods5040080. PMID: 28231175; PMCID: PMC5302426.
- Jayachandran M, Xiao J, Xu B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int J Mol Sci. 2017 Sep 8;18(9):1934. doi: 10.3390/ijms18091934. PMID: 28885559; PMCID: PMC5618583.
- Darge HF, Andrgie AT, Tsai HC, Lai JY. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int J Biol Macromol. 2019 Jul 15;133:545-563. doi: 10.1016/j.ijbiomac.2019.04.131. Epub 2019 Apr 18. PMID: 31004630.
- Yang L, Zhang LM. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr Polym. 2009; 76:349–361. doi: 10.1016/j.carbpol.2008.12.015.
- Zhang Y, Wang F. Carbohydrate drugs: current status and development prospect. Drug Discov Ther. 2015 Apr;9(2):79-87. doi: 10.5582/ddt.2015.01028. PMID: 25994058.
- Ngwuluka NC. Responsive polysaccharides and polysaccharides-based nanoparticles for drug delivery. Amsterdam: Elsevier; 2018.
- Krishtanova NA, Safonova MYu, Bolotova VTs. Prospects for the use of plant polysaccharides as therapeutic and therapeutic and prophylactic agents // Bulletin of the VSU. Series: Chemistry. Biology. Pharmacy. 2005; 1: S212-221.
- Li C, Li X, You L, Fu X, Liu RH. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr Polym. 2017 Jan 2;155:261-270. doi: 10.1016/j.carbpol.2016.08.075. Epub 2016 Aug 25. PMID: 27702511.
- Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr Polym. 2016 Sep 5;148:86-97. doi: 10.1016/j.carbpol.2016.02.060. Epub 2016 Apr 13. PMID: 27185119.
- Shestopalova NN. The study of polysaccharides of the grass Acroptilon repens L. of the flora of the Tula region // Scientific result. Medicine and pharmacy. 2018; 4: S70-76. DOI: 10.18413/2313-8955-2018-4-1-70-76.
- Jahangir M, Kim HK, Choi YH, Verpoorte R. Comprehensive Reviews in Food Science and Food Safety. 2009; 8: 31–43. DOI: 10.1111/j.1541-4337.2008.00065. x.
- Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002 Sep;76(3):560-8. doi: 10.1093/ajcn/76.3.560. PMID: 12198000.
- Xie Y, Jiang S, Su D, Pi N, Ma C, Gao P. Composition analysis and anti-hypoxia activity of polysaccharide from Brassica rapa L. Int J Biol Macromol. 2010 Nov 1;47(4):528-33. doi: 10.1016/j.ijbiomac.2010.07.008. Epub 2010 Aug 3. PMID: 20678519.
- Baenas N, Moreno DA, García-Viguera C. Selecting sprouts of brassicaceae for optimum phytochemical composition. J Agric Food Chem. 2012 Nov 14;60(45):11409-20. doi: 10.1021/jf302863c. Epub 2012 Nov 1. PMID: 23061899.
- Sousa C, Taveira M, Valentão P, Fernandes F, Pereira JA, Estevinho L, Bento A, Ferreres F, Seabra RM, Andrade PB. Inflorescences of Brassicacea species as source of bioactive compounds: A comparative study. Food Chem. 2008 Oct 15;110(4):953-61. doi: 10.1016/j.foodchem.2008.02.087. Epub 2008 Mar 8. PMID: 26047285.
- Romani A, Vignolini P, Isolani L, Ieri F, Heimler D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. Subsp. sylvestris L.). J Agric Food Chem. 2006 Feb 22;54(4):1342-6. doi: 10.1021/jf052629x. PMID: 16478258.
- Xue YL, Han HT, Liu CJ, Gao Q, Li JH, Zhang JH, Li DJ, Liu CQ. Multivariate analyses of the volatile components in fresh and dried turnip (Brassica rapa L.) chips via HS-SPME-GC-MS. J Food Sci Technol. 2020 Sep;57(9):3390-3399. doi: 10.1007/s13197-020-04372-y. Epub 2020 Apr 3. PMID: 32728286; PMCID: PMC7374492.
- Paul S, Geng CA, Yang TH, Yang YP, Chen JJ. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J Food Sci. 2019 Jan;84(1):19-30. doi: 10.1111/1750-3841.14417. Epub 2018 Dec 18. PMID: 30561035.
- Aires A, Fernandes C, Carvalho R, Bennett RN, Saavedra MJ, Rosa EA. Seasonal effects on bioactive compounds and antioxidant capacity of six economically important brassica vegetables. Molecules. 2011 Aug 10;16(8):6816-32. doi: 10.3390/molecules16086816. PMID: 21832972; PMCID: PMC6264194.
- Paul S, Zhang X, Yang Y, Geng C. Chemical constituents from turnip and their effects on α-glucosidase. Phyton. 2020; 89:131-136. 10.32604/phyton.2020.08328
- Li Y, Han J, Chen Y, Chen C, Chu B, Zhang Y. p-Coumaric acid as a prophylactic measure against normobaric hypoxia induced pulmonary edema in mice. Life Sci. 2018 Oct 15;211:215-223. doi: 10.1016/j.lfs.2018.09.039. Epub 2018 Sep 22. PMID: 30248349.
- Li Y, Han J, Zhang Y, Chen Y, Zhang Y. Prophylactic effect and mechanism of p-coumaric acid against hypoxic cerebral edema in mice. Respir Physiol Neurobiol. 2019 Feb;260:95-104. doi: 10.1016/j.resp.2018.11.004. Epub 2018 Nov 14. PMID: 30447305.
- El-Makawy AI, Ibrahim FM, Mabrouk DM, Abdel-Aziem SH, Sharaf HA, Ramadan MF. Efficiency of turnip bioactive lipids in treating osteoporosis through activation of Osterix and suppression of Cathepsin K and TNF-α signaling in rats. Environ Sci Pollut Res Int. 2020 Jun;27(17):20950-20961. doi: 10.1007/s11356-020-08540-7. Epub 2020 Apr 6. PMID: 32253695.
- Staub AM. Removeal of Protein-Sevag Method. Methods in Carbohydrate. Chemistry. 1965; 5: 5-6.
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analitical Chemistry. 1956; 28: 350-356. DOI: 10.1021/ac60111a017.
- Decree of the Cabinet of Ministers of the Republic of Uzbekistan No. 213 dated March 23, 2018, “On approval of the regulation on the procedure for state registration of medicines, medical devices and medical equipment and the issuance of a registration certificate” (in Uzbek).
- Guidelines for conducting preclinical studies of drugs. Part one. Ed. Mironov AN. Moscow: Grif and K, 2012; 944.
- Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use, ICH guidelines S2(R1). 2011. https://database.ich.org/sites/default/files/S2%28R1%29%20Guideline.pdf.
- Directive 2010/63/EU on the protection of animals used for scientific purposes. September 22, 2010. Official Journal of the European Union, L 276/33- L276/79.
- Sheriff OL, Olayemi O, Taofeeq AO, Riskat KE, Ojochebo DE, Ibukunoluwa AO. A new model for alloxan-induced diabetes mellitus in rats. J Bangladesh Society of Physiologist. 2020; 14: 56–62. DOI:10.3329/jbsp.v14i2.44785
- Pansare K, Upasani C, Upangalwar A, Sonawane G, Patil C. Streptozotocin and Alloxan Induced Diabetic Nephropathy: Protective Role of Natural Products. J Maharaja Sayajirao Univ Baroda. 2021; 55: 86–102.
- Kim YH, Kim YW, Oh YJ, Back NI, Chung SA, Chung HG, Jeong TS, Choi MS, Lee KT. Protective Effect of the Ethanol Extract of the Roots of Brassica rapa on Cisplatin-Induced Nephrotoxicity in LLC-PK1 Cells and Rats. Biological Pharmaceutical Bulletin. 2006; 29: 2436-2441. DOI: 10.1248/bpb.29.2436.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
