International Journal of Pharmaceutical Sciences and Developmental Research
Synergistic antifungal effectiveness of essential oils from andean plants combined with commercial drugss
Beatriz Lima1,2, Maximiliano Sortino2-4, Alejandro Tapia1 and Gabriela E Feresin1,2*
2National Council for Scientific and Technical Research (CONICET), Godoy Cruz 2290 (C1425FQB) CABA – Argentina
3Pharmacognosy, Faculty of Biochemical and Pharmaceutical Sciences, National University of Rosario, Suipacha 531, 2000 Rosario, Argentina
4Mycology Reference Center, Faculty of Biochemical and Pharmaceutical Sciences, National University of Rosario, Suipacha 531, 2000 Rosario, Argentina
Cite this as
Lima B, Sortino M, Tapia A, Feresin GE (2022) Synergistic antifungal Effectiveness of essential Oils from andean plants combined with commercial drugs. Int J Pharm Sci Dev Res 8(1): 023-031. DOI: 10.17352/ijpsdr.000039Copyright License
© 2022 Lima B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The appearance of antifungal resistance promotes the investigation of therapeutic options. There are few studies on the combined effect of antifungal drugs and essential oils (EOs). In the present work, regarding the association of eight EOs Andean plants with antifungal agents against a panel of fungi strains. Combinatorial effects were determined using the Fractional Inhibitory Concentration Index (FICI) and Dose Reduction Index (DRI). A combination of A. cryptantha-B EO with fluconazole showed a synergistic effect against C. Albicans (FIC = 0.31 and DRI = 16.25). EOs from A. cryptantha-A and L. integrifolia showed an additive effect (FICI = 0.75) against C. neoformans. A combination of EOs from A. seriphioides and A. cryptantha-A with terbinafine showed an additive effect on T. rubrum (FIC = 0.56; DRI = 16) and M. gypseum (FICI = 1.03; DRI = 32). In conclusion, combinations between EOs of species from Andean plants and commercial antifungal drugs yielded some interesting findings, as potential antifungal strategies used for treating infections associated with C. Albicans and T. rubrum .
Introduction
Around the world, the number of deaths from fungal infections significantly increases every year. The most susceptible age groups are the elderly, premature newborns, and individuals with deficient immune systems. Additionally, prolonged and prophylactic therapeutic use of antifungal drugs promotes the emergence of drug-resistant Microorganisms [1]. Fungal diseases cause dermatophytosis, subcutaneous mycoses, systemic mycoses, and other mycoses. In particular, Candida albicans fungi are one of the leading causes of superficial infections of the skin, mouth, and mucous membranes, as well as life-threatening systemic infections [2,3].
Another clinically important fungus that affects immunocompromised patients is Cryptococcus neoformans. Moreover, cryptococcosis in positive HIV/AIDS patients has become a leading cause of death [4-6]. These infections are treated by drugs that mainly contain fluconazole (FLC) due to its high efficacy and low toxicity, a consequence of its high-water solubility and low affinity for plasma proteins. Nevertheless, its inadequate and long-term use produces multidrug-resistant strains that strongly affect the effciency of commercial drugs [7-9]. On the other hand, dermatophytes are species of fungi that typically infect and invade a living host’s skin, hair, and nails. These benign, common infections are caused by the genera Trichophyton, Microsporum, and Epidermophyton, and are usually limited to the stratum corneum or keratinized adnexal structures [10-12].
In some parts of the world, dermatophytoses are found easy to treat, although recurrent infections such as tinea corporis and cruris need more extensive treatments. Antifungal agents like imidazoles and allylamines, namely terbinafine, are very effective but can cause severe toxicity [13]. This situation demands an understanding of the pharmacokinetic and pharmacodynamic properties of the commercially available effective drugs against dermatophytes and an analysis of the factors that may be contributing to drug resistance [14]. Antifungal combination therapy is one of the strategies used to fight the resistance of fungi, caused by the limited therapeutic efficiency of the existing commercial antifungals [15]. Moreover, different authors emphasize the need of combining commercial drugs with natural bioactive products to reduce their toxicity and decrease the mortality index caused by inadequate and extensive use of antimicrobials [16-18]. Combination therapy produces different effects, such as synergistic, additive, indifferent, and antagonistic results. These can be equal, weaker, or stronger than that of the use of an individual drug per se [19]. A recent review showed that essential oils (EOs) have a variety of different properties (antiviral, nematocidal, antimicrobial, insecticidal, and antioxidant), due to the presence of diverse bioactive molecules [20]. Therefore, EOs could become important partners for antifungal drugs [21,22]. The main goal of this study was to evaluate the synergistic antifungal effects (FICI ≤ 0.5) of the combination between eight EOs from Acantholippia seriphioides Gray Mold, Azorella cryptantha (Clos) Reiche (A and B) Clinopodium gilliesii (Benth.) Kuntze, Gymnophyton polycephalum Gillies & Hook, Clos., Lippia integrifoliaGriseb Hieron, Seriphidium mendozanum (DC.) K. Bremer & Humphries, and Tagetes mendocina Phil, and the commercial drugs fluconazole (FLC) and terbinafine (TRB), on the fungi C. Albicans , C. neoformans, M. gypseum, and T. rubrum .
Materials and methods
Chemicals and materials
RPMI-1640 culture medium (Sigma-Aldrich, St. Louis, MO, USA) was buffered to pH 7.0 with 3-(N-morpholino) propane sulfonic acid (MOPS) (Sigma-Aldrich). Fluconazole (FLC) and terbinafine (TRB) were obtained from Sigma-Aldrich. Dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany).
Plant material
The following plant species were used: Acantholippia seriphioides (A. Gray) Moldenke (Verbenaceae), voucher number (vn) BT-39; Azorella cryptantha (Clos) Reiche (Apiaceae) collected at 2700 m.a.s.l., vn CORD 1193 (collection A. cryptantha-A) and collected at 4000 m.a.s.l., vn CORD 1188 (collection A. cryptantha-B); Clinopodium gilliesii (Benth.) Kuntze (Asteraceae), vn BT-7; Gymnophyton polycephalum Gillies & Hook, Clos. (Apiaceae), vn BT-41; Lippia integrifolia(Griseb.) Hieron (Verbenaceae), vn BT-40; Seriphidium mendozanum (DC.) K. Bremer & Humphries (Asteraceae), vn BT-6; and Tagetes mendocina Phil. (Asteraceae), vn BT-5.
Essential oil extraction
Fresh aerial parts (1000 g) were subjected to hydrodistillation (2 h) using a Clevenger-type apparatus according to The European Pharmacopoeia [23]. EOs yields (% w/v) were the following: A. seriphioides (0.75), A. cryptantha-A (1.0), A. cryptantha-B (0.4), Clinopodium gilliesii (2.40), G. polycephalum (1.15), L. integrifolia (1.50), S. mendozanum (0.62), T. mendocina (0.81% w/v). EOs were stored at -18°C until used in the antifungal combinatory studies.
Antifungal activity assay
Microorganisms: Candida albicans (ATCC 10231), Cryptococcus neoformans (ATCC 32264), Trichophyton rubrum CCC110 and Microsporum gypseum CCC115, were provided by the Centro de Referencia Micólogica CEREMIC (CCC), Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Argentina. Minimum inhibitory concentration (MIC). EOs and commercial drugs were determined by the microdilution technique according to Lima, et al. [24]. The serial dilutions of EOs (final concentration of 1000 to 0.98 μg/ml) and commercial drugs (final concentration of 50 to 0.98 μg/ml) were obtained starting from stock solutions in DMSO to a final concentration ≤1%) and were added to the medium achieving a final volume of 100 μl. Endpoints were defined as the lowest concentration of EOs or commercial drugs resulting in total inhibition (MIC) compared to growth controls (culture media and Microorganisms alone) and were determined spectrophotometrically at 405 nm using a VERSA Max microplate reader (Molecular Devices, USA).
In vitro antifungal combinatory effect between EOs and commercial drug
Checkerboard design: The checkerboard design was used to assess the combinatory effect between EOs and FLC and ITC on yeasts and of EOs and TRB on dermatophytes, as described by Gomez, et al. [25]. The fractional inhibitory concentration (FIC) and fractional inhibitory concentration index (FICI) were calculated using the following equations:
FICEO = MICEO alone/MICEOalone (1)
FIC ANT = MICANT comb / MICANTalone (2)
FICI = FICEO+FICANT (3)
The results were interpreted as follows: synergistic effect (FICI ≤ 0.5), additive effect (0.5 > FICI< 2.0), no interaction or indifference (2 ≥ FICI < 4), and antagonistic effect (FICI > 4) as described by Nikolić, et al. [26].
Dose Reduction Index (DRI)
The dose reduction index (DRI) is a measure of how many times the dose of each antifungal drug in a synergistic combination may be reduced to a given effect level compared to the doses of each individual substance. A higher value of DRI (>1) indicates a greater dose reduction for a given effect level [27,28]. The DRI value for each corresponding drug is calculated as follows:
DRI = MIC x alone / MIC x in the comb (4)
Isobolograms
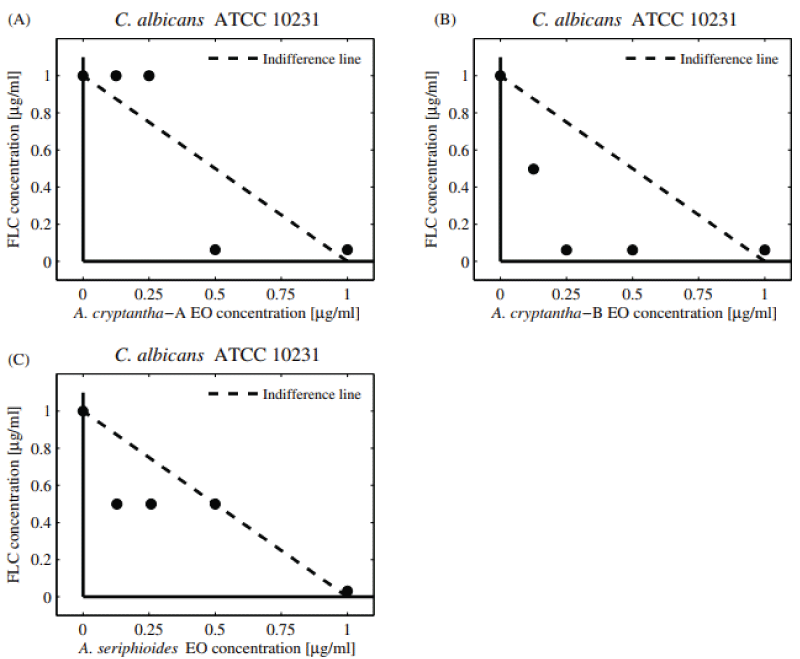
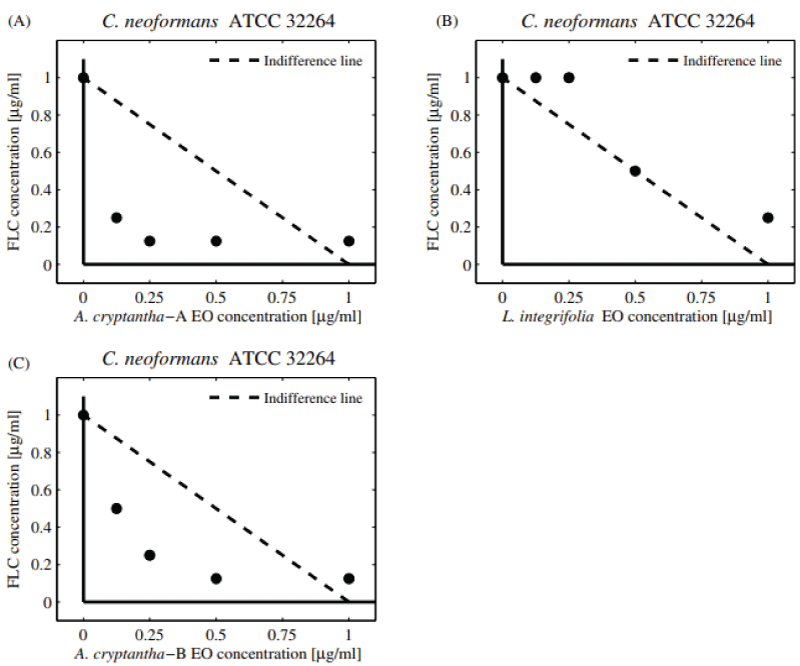
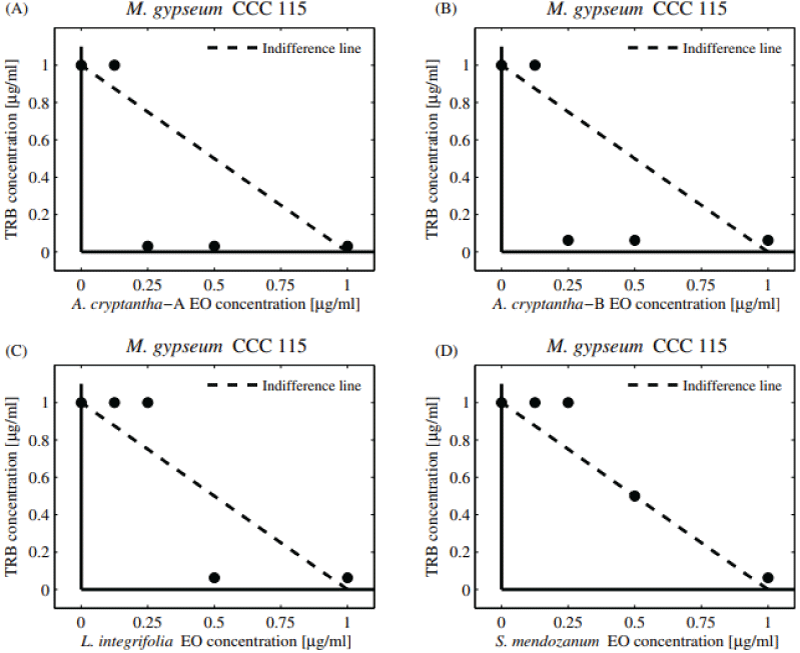
The potential antifungal combinatory effects between EOs and commercial drugs are presented in normalized isobolograms based on the results of the checkerboard design (Supplementary Figure 1). In the isobologram, the x-axis and y-axis represent EOs and commercial antifungals, respectively. The MIC value of EOs alone is located on the x-axis and the MIC value of the antifungal drug alone is on the y-axis. The line connecting these two points represents the line of no interaction (indifference line). The MIC values obtained below the line indicate a synergistic effect (FICI ≤ 0.5), whereas those above the line are explained as an antagonistic effect (FICI > 4) [29].
Results and discussion
An increase in microbial resistance to existing drugs has sparked a surge of global interest in antimicrobial natural products originating from plants such as EOs. Aljaafari, et al. [30] reported the effect of EOs combined with other available antifungal agents is a promising strategy for increasing antifungal efficacy and/or decreasing the toxicity of commercial antifungal agents.
Antifungal activity
Table 1 shows the antifungal effect of the EOs of Andean plants from A. seriphioides , A. cryptantha-A, A. cryptantha-B, and Clinopodium gilliesii showed the greatest activity against C. Albicans with MIC values between 250-500 μg/ml. The C. neoformans strain was strongly inhibited by the EO of A. cryptantha-B with a MIC value of 125 μg/ml, but lower effects were observed with A. cryptantha-A and Clinopodium gilliesii EOs (MIC = 250 μg/ml).
Regarding dermatophytes, M. gypseum was sensitive to EOs from A. cryptantha-A, A. seriphioides , L. integrifolia , and T. mendocina (MIC = 250 μg/ml), whereas T. rubrum was inhibited by EOs from S. mendozanum , A. seriphioides , A. cryptantha (A and B), Clinopodium gilliesii and T. mendocina EOs with MIC values between 250-500 μg/ml. Chemically, EOs are a mixture of terpenes, phenylpropanoids, and terpenoids. Their reactivity against different pathogens is based on the functional groups they possess [20,30]. EOs of A. seriphioides , S. mendozanum , and both collections of A. cryptantha showed the greatest combinatory antifungal effect with the selected commercial drugs. Previous studies reported the chemical composition of the EOs from these species collected in the central Andes region of Argentina [24,31]. The main components of the EO from A. seriphioides are p-cymene, γ-terpinene, and a high content of bioactive oxygenated monoterpenes such as thymol (27.61%) and carvacrol (13.24%) [24]. Thymol is a naturally occurring phenol monoterpene derived from cymene and an isomer of carvacrol.
The multicomponent composition of EOs (usually comprised of twenty to sixty constituents in varying quantities), generates simultaneous activities and presents advantages compared to commercial drugs. The reactivity against different pathogens is related to their functional groups of them [30]. These complex mixed mechanisms of action make it more difficult to develop fungal resistance compared with a single target therapy. EOs deploys effects against pathogen fungi, interrupting cell communication and mycotoxin synthesis which in turn debilitates fungal growth [32,33]. Ahmad, et al. [34] reported interactions between the components of essential oils that resulted in synergistic combinations. Thymol and carvacrol alter permeability, causing the release of cellular components from the cells. In addition, these terpenoids modify cellular processes such as DNA transcription, protein synthesis, and enzyme activity, affecting, for example, the enzymes producing ATP and thus reducing intracellular ATP levels. γ-terpinene promotes the entry of other molecules via the membrane, which then act on their respective targets. The combination of terpinenes with terpenoids increases the amount and size of the pores through a synergistic interaction between them [35,36]. Thymol was found to have a fungicidal effect on Candida species and exhibited a synergistic effect combined with nystatin (FICI = 0.25) [37]. Nazzaro, et al. [33] reported that the combination of carvacrol and fluconazole exhibited a significant synergistic effect, inhibiting the over-expression of efflux-pump drug-resistance genes CDR1 (Candida drug resistance) and MDR1 (multidrug resistance) in C. Albicans which inhibited efflux by 70% - 90%, thus evidencing a great potential to block drug transporter pumps. Also, Braga, et al. [38] evaluated the synergistic effect of eugenol and thymol and their mechanism of action showed morphological alterations in the membrane of C. Albicans . Jafri and Ahmad [39] reported that antifungal drug activity is greatly increased in the presence of thymol on C. Albicans and C. tropicalis strains. In addition, thymol resulted in an alternative agent for the treatment of biofilm-associated with C. Albicans and C. tropicalis infections. Shaban, et al. [40] showed evidence of enhanced antifungal activity for a combination of oxygenated compounds with antifungal drugs against Candida Auris, where the interaction of carvacrol was evidenced as antifungal and anti-virulent. Also, carvacrol effectively reduced the gene expression of secreted aspartyl proteinase (SAP), generating a higher effect against C. Albicans isolates [41]. Previous reports described the chemical composition of the EOs of S. mendozanum , in which the main actives terpenes are camphor, borneol, astemizole, and artemisia alcohol [42]. On the other hand, although the chemical profile of sesquiterpene hydrocarbons in the EOs of both collections of A. cryptantha was similar, there were variations in the relative proportion of the main components such as the content of oxygenated monoterpenes (3.0% total) as cis-β-terpineol, isoborneol, and borneol present in A. cryptantha-B EO [43]. The antifungal effect of borneol and its derivates against C. Albicans yeast has been previously reported [44]. Also, borneol, thymol, camphor, and isoborneol are responsible for the anti-dermatophyte efficacy of EOs [45,46].
In vitro antifungal combinatory effect between EOs and antifungal drugs
The antifungal combinatory effect (determination of FIC and FICI) between FLC and selected EOs against yeasts is shown in Table 2 and Figures 1,2. The EOs of A. cryptantha-B with FLC showed a synergistic effect on C. Albicans , (FICI = 0.31, Equations 1-3; DRI = 16.25, Equation 4), while the EO of A. cryptantha-A and A. seriphioides elicited an additive effect (FICI = 1.03; DRI = 32.50 and FICI = 1.49). The results represented in the normalized isobolograms (Figure 1A-C) agree with those obtained by the checkerboard analysis. The combination of A. cryptantha-B EO and FLC evidenced a synergistic effect on C. Albicans , as shown by the resulting values of the experimental combinations which are all below the line of indifference (Figure 1A). On the other hand, an additive effect was observed for the EOs from A. cryptantha-A and A. seriphioides EOs evaluated against this strain (Figure 1B, C).
Regarding the effects on C. neoformans, the combination between S. mendozanum EO and FLC showed an additive effect (FICI = 0.625; DRI = 2), (Figure 2A-D). A similar effect was observed with the EOs of A. cryptantha-A, L. integrifolia (FICI = 0.75; DRI = 4), and A. cryptantha-B (FICI = 1; DRI = 2). Only the EOs from A. cryptantha A and B showed additive interactions as shown by the experimental values of FICI which are all below the line of indifference Figures 2B, D.
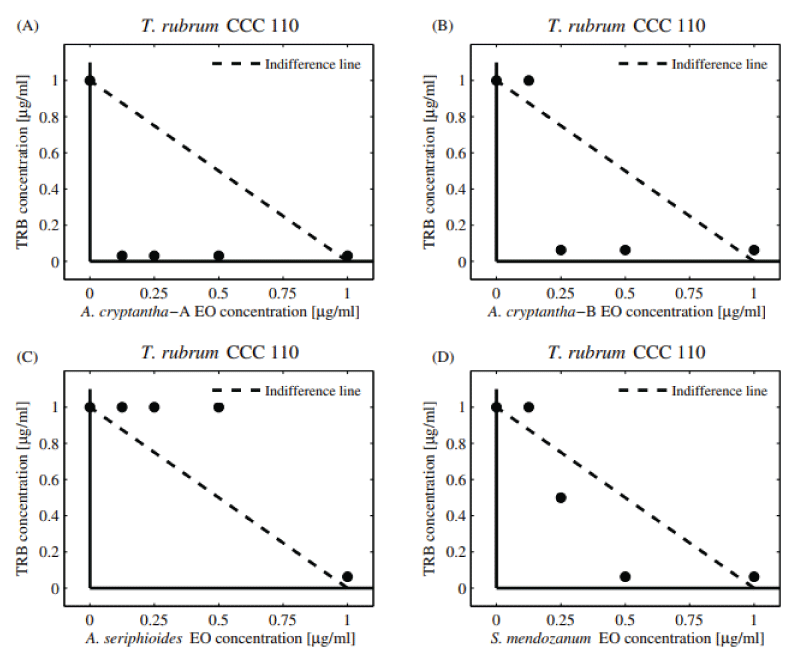
Table 3 shows the combinatory effect (FIC and FICI) of TRB and EOs against dermatophytes M. gypseum and T. rubrum . Additive interactions were observed against M. gypseum (FICI = 1.03-1.06). The significant decrease observed in the dose of TRB (DRI = 16-32) could be largely due to the adjuvant property of the compounds present in the EOs coinciding with the available literature [26]. Regarding T. rubrum , additive interactions were observed when combining the EOs of A. seriphioides , and A. cryptantha-B and TRB, obtaining a “borderline case of synergism” (FICI = 0.56, DRI = 16). The five EOs extracted from S. mendozanum , A. cryptantha-A, L. integrifolia , C. gilliessi, and T. mendocina displayed additive activity (FICI = 1.03-1.06). Moreover, a pronounced decrease in the individual MIC value of TRB was observed (DRI = 16-32).
Additive activity can be observed in the normalized isobolograms (Figures 3A-D and 4A-D) with combination points below (both collections of A. cryptantha EOs) and above the indifference line against both dermatophytes strains tested. The dermatophytes showed low sensitivity to the combinations tested.
Conclusion
The highest antifungal properties reported for carvacrol, and thymol, indicate that these main components in the EOs assayed, and especially to some combinations EOs selected with commercial antifungals provided benefits, including synergistic effect and reduced toxic effects, showing their potential to treat infections associated to C. Albicans and T. rubrum . The present study shows the antifungal effect of EOs from Andean plants, containing the active ingredients borneol, carvacrol, and thymol, against relevant fungal strains such as yeasts (Candida albicans , Cryptococcus neoformans) and dermatophytes (T. rubrum and M. gypseum). Both collections of A. cryptantha (A and B) and A. seriphioides EOs yielded some interesting findings; they were the most promising combinations that show efficacy against pathogenic fungi. Finally, these results confirm the importance of carrying out further studies on combined strategies using natural products and commercial drugs to improve the increasing problem of antibiotic resistance.
The authors are grateful to, PIO-CONICET-SECITI Nº 0022 and CICITCA, UNSJ, Argentina. M S, G.E. F., and B.L. are researchers from CONICET, Argentina.
- Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D. Fungal infections in humans: the silent crisis. Microb Cell. 2020 Jun 1;7(6):143-145. doi: 10.15698/mic2020.06.718. PMID: 32548176; PMCID: PMC7278517.
- Sellam A, Whiteway M. Recent advances on Candida albicans biology and virulence. F1000Res. 2016 Oct 26;5:2582. doi: 10.12688/f1000research.9617.1. PMID: 27853524; PMCID: PMC5089126.
- Chen H, Zhou X, Ren B, Cheng L. The regulation of hyphae growth in Candida albicans . Virulence. 2020 Dec;11(1):337-348. doi: 10.1080/21505594.2020.1748930. PMID: 32274962; PMCID: PMC7161696.
- Denning DW. Minimizing fungal disease deaths will allow the UNAIDS target of reducing annual AIDS deaths below 500 000 by 2020 to be realized. Philos Trans R Soc Lond B Biol Sci. 2016 Dec 5;371(1709):20150468. doi: 10.1098/rstb.2015.0468. PMID: 28080991; PMCID: PMC5095544.
- Samie S, Trollope KM, Joubert LM, Makunga NP, Volschenk H. The antifungal and Cryptococcus neoformans virulence attenuating activity of Pelargonium sidoides extracts. J Ethnopharmacol. 2019 May 10;235:122-132. doi: 10.1016/j.jep.2019.02.008. Epub 2019 Feb 6. PMID: 30738119.
- Hu S, Gu F, Chen M, Wang C, Li J, Yang J, Wang G, Zhou Z, Yang Y. A novel method for identifying and distinguishing Cryptococcus neoformans and Cryptococcus gattii by surface-enhanced Raman scattering using positively charged silver nanoparticles. Sci Rep. 2020 Jul 27;10(1):12480. doi: 10.1038/s41598-020-68978-0. PMID: 32719360; PMCID: PMC7385644.
- Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem. 2012 Oct 1;20(19):5678-98. doi: 10.1016/j.bmc.2012.04.045. Epub 2012 May 9. Erratum in: Bioorg Med Chem. 2013 Feb 1;21(3):834. Erratum in: Bioorg Med Chem. 2013 Mar 1;21(5):1367. PMID: 22902032.
- Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int. 2013;2013:204237. doi: 10.1155/2013/204237. Epub 2013 Jun 26. PMID: 23878798; PMCID: PMC3708393.
- Morace G, Perdoni F, Borghi E. Antifungal drug resistance in Candida species. J Glob Antimicrob Resist. 2014 Dec;2(4):254-259. doi: 10.1016/j.jgar.2014.09.002. Epub 2014 Sep 28. PMID: 27873684.
- AL-Khikani FH. Dermatophytosis a worldwide contiguous fungal infection: Growing challenge and few solutions. Biomed Biotechnol Res J. 2020; 4:117-122.
- Rouzaud C, Hay R, Chosidow O, Dupin N, Puel A, Lortholary O, Lanternier F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J Fungi (Basel). 2015 Dec 31;2(1):4. doi: 10.3390/jof2010004. PMID: 29376922; PMCID: PMC5753085.
- Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs Context. 2020 Jul 20;9:2020-5-6. doi: 10.7573/dic.2020-5-6. PMID: 32742295; PMCID: PMC7375854.
- Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008 Nov-Dec;166(5-6):353-67. doi: 10.1007/s11046-008-9109-0. Epub 2008 May 14. PMID: 18478357.
- Khurana A, Sardana K, Chowdhary A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet Biol. 2019 Nov;132:103255. doi: 10.1016/j.fgb.2019.103255. Epub 2019 Jul 19. PMID: 31330295.
- Liu Y, Ren H, Wang D, Zhang M, Sun S, Zhao Y. The synergistic antifungal effects of gypenosides combined with fluconazole against resistant Candida albicans via inhibiting the drug efflux and biofilm formation. Biomed Pharmacother. 2020 Oct;130:110580. doi: 10.1016/j.biopha.2020.110580. Epub 2020 Jul 31. PMID: 32745913.
- Azevedo RVDM, Rizzo J, Rodrigues ML. Virulence Factors as Targets for Anticryptococcal Therapy. J Fungi (Basel). 2016 Nov 30;2(4):29. doi: 10.3390/jof2040029. PMID: 29376946; PMCID: PMC5715936.
- He B, Lu C, Zheng G, He X, Wang M, Chen G, Zhang G, Lu A. Combination therapeutics in complex diseases. J Cell Mol Med. 2016 Dec;20(12):2231-2240. doi: 10.1111/jcmm.12930. Epub 2016 Sep 7. PMID: 27605177; PMCID: PMC5134672.
- Campitelli M, Zeineddine N, Samaha G, Maslak S. Combination Antifungal Therapy: A Review of Current Data. J Clin Med Res. 2017 Jun;9(6):451-456. doi: 10.14740/jocmr2992w. Epub 2017 Apr 26. PMID: 28496543; PMCID: PMC5412516.
- Cokol M, Chua HN, Tasan M, Mutlu B, Weinstein ZB, Suzuki Y, Nergiz ME, Costanzo M, Baryshnikova A, Giaever G, Nislow C, Myers CL, Andrews BJ, Boone C, Roth FP. Systematic exploration of synergistic drug pairs. Mol Syst Biol. 2011 Nov 8;7:544. doi: 10.1038/msb.2011.71. PMID: 22068327; PMCID: PMC3261710.
- Abd Rashed A, Rathi DG, Ahmad Nasir NAH, Abd Rahman AZ. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules. 2021 Feb 19;26(4):1093. doi: 10.3390/molecules26041093. PMID: 33669627; PMCID: PMC7922942.
- Rai M, Paralikar P, Jogee P, Agarkar G, Ingle AP, Derita M, Zacchino S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int J Pharm. 2017 Mar 15;519(1-2):67-78. doi: 10.1016/j.ijpharm.2017.01.013. Epub 2017 Jan 9. PMID: 28089935.
- Rezgui M, Majdoub N, Mabrouk B, Baldisserotto A, Bino A, Ben Kaab LB, Manfredini S. Antioxidant and antifungal activities of marrubiin, extracts and essential oil from Marrubium vulgare L. against pathogenic dermatophyte strains. J Mycol Med. 2020 Apr;30(1):100927. doi: 10.1016/j.mycmed.2020.100927. Epub 2020 Jan 11. PMID: 31983544.
- Council of Europe (COE) – European Directorate for the Quality of Medicines, in European Pharmacopeia, 5th ed, Strasbourg, 2005, 1: 217.
- Lima B, López S, Luna L, Agüero MB, Aragón L, Tapia A, Zacchino S, López ML, Zygadlo J, Feresin GE. Essential oils of medicinal plants from the central andes of Argentina: chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chem Biodivers. 2011 May;8(5):924-36. doi: 10.1002/cbdv.201000230. PMID: 21560241.
- Gómez J, Simirgiotis MJ, Manrique S, Piñeiro M, Lima B, Bórquez J, Feresin GE, Tapia A. UHPLC-ESI-OT-MS Phenolics Profiling, Free Radical Scavenging, Antibacterial and Nematicidal Activities of "Yellow-Brown Resins" from Larrea spp. Antioxidants (Basel). 2021 Jan 28;10(2):185. doi: 10.3390/antiox10020185. PMID: 33525584; PMCID: PMC7911333.
- Nikolić MM, Jovanović KK, Marković TL, Marković DL, Gligorijević NN, Radulović SS, Kostić M, Glamočlija JM, Soković MD. Antimicrobial synergism and cytotoxic properties of Citrus limon L., Piper nigrum L. and Melaleuca alternifolia (Maiden and Betche) Cheel essential oils. J Pharm Pharmacol. 2017 Nov;69(11):1606-1614. doi: 10.1111/jphp.12792. Epub 2017 Aug 17. PMID: 28815601.
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006 Sep;58(3):621-81. doi: 10.1124/pr.58.3.10. Erratum in: Pharmacol Rev. 2007 Mar;59(1):124. PMID: 16968952.
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010 Jan 15;70(2):440-6. doi: 10.1158/0008-5472.CAN-09-1947. Epub 2010 Jan 12. PMID: 20068163.
- Gessner PK. Isobolographic analysis of interactions: an update on applications and utility. Toxicology. 1995 Dec 28;105(2-3):161-79. doi: 10.1016/0300-483x(95)03210-7. PMID: 8571354.
- Aljaafari MN, AlAli AO, Baqais L, Alqubaisy M, AlAli M, Molouki A, Ong-Abdullah J, Abushelaibi A, Lai KS, Lim SE. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules. 2021 Jan 26;26(3):628. doi: 10.3390/molecules26030628. PMID: 33530290; PMCID: PMC7866131.
- López S, Lima B, Aragón L, Espinar LA, Tapia A, Zacchino S, Zygadlo J, Feresin GE, López ML. Essential oil of Azorella cryptantha collected in two different locations from San Juan Province, Argentina: chemical variability and anti-insect and antimicrobial activities. Chem Biodivers. 2012 Aug;9(8):1452-64. doi: 10.1002/cbdv.201100319. PMID: 22899606.
- Böhme K, Barros-Velázquez J, Calo-Mata P, Aubourg SP. Chapter 3: ‘Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds: Current Strategies and New Alternatives’ Villa, T.G., Veiga-Crespo, P., Eds.; Springer: Berlin/Heidelberg, Germany, 51–81. 2014.
- Nazzaro F, Fratianni F, Coppola R, Feo V. Essential Oils and Antifungal Activity. Pharmaceuticals (Basel). 2017 Nov 2;10(4):86. doi: 10.3390/ph10040086. PMID: 29099084; PMCID: PMC5748643.
- Ahmad A, van Vuuren S, Viljoen A. Unravelling the complex antimicrobial interactions of essential oils--the case of Thymus vulgaris (thyme). Molecules. 2014 Mar 6;19(3):2896-910. doi: 10.3390/molecules19032896. PMID: 24662066; PMCID: PMC6271043.
- Cristani M, D'Arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, Venuti V, Bisignano G, Saija A, Trombetta D. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem. 2007 Jul 25;55(15):6300-8. doi: 10.1021/jf070094x. Epub 2007 Jun 30. PMID: 17602646.
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012 Jan 25;3:12. doi: 10.3389/fmicb.2012.00012. PMID: 22291693; PMCID: PMC3265747.
- de Castro RD, de Souza TM, Bezerra LM, Ferreira GL, Costa EM, Cavalcanti AL. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: an in vitro study. BMC Complement Altern Med. 2015 Nov 24;15:417. doi: 10.1186/s12906-015-0947-2. PMID: 26601661; PMCID: PMC4659158.
- Braga PC, Sasso MD, Culici M, Alfieri M. Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans . Fitoterapia. 2007 Sep;78(6):396-400. doi: 10.1016/j.fitote.2007.02.022. Epub 2007 May 23. PMID: 17590533.
- Jafri H, Ahmad I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J Mycol Med. 2020 Apr;30(1):100911. doi: 10.1016/j.mycmed.2019.100911. Epub 2019 Nov 7. PMID: 32008964.
- Shaban S, Patel M, Ahmad A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci Rep. 2020 Jan 24;10(1):1162. doi: 10.1038/s41598-020-58203-3. PMID: 31980703; PMCID: PMC6981193.
- Hosseini SS, Yadegari MH, Rajabibazl M, Ghaemi EA. Inhibitory effects of carvacrol on the expression of secreted aspartyl proteinases 1-3 in fluconazole-resistant Candida albicans isolates. Iran J Microbiol. 2016 Dec;8(6):401-409. PMID: 28491252; PMCID: PMC5420396.
- Lima B, de Lampasona MP, Schuff C, Tapia A, Bomben R, Duschatzky C, Feresin GE. Chemical composition and antibacterial activity of Artemisia mendozana D.C. essential oil. J Ess Oil Bearing Plants. 2008; 11: 496-502.
- López S, Lima B, Aragón L, Ariza Espinar L, Tapia A, Zacchino S, Zygadlo J, Feresin GE, López ML. Essential oil Azorella cryptantha collected in two different locations from San Juan province, Argentina. Chemical variability anti-insect, and antimicrobial activities. Chem Biodivers. 9: 1452-1464. http://doi.org/10.1002/cbdv.201100319
- Al-Farhan KA, Warad I, Al-Resayes SI, Fouda MM, Ghazzali M. Synthesis, structural chemistry and antimicrobial activity of -(-) borneol derivative. Central European J of Chem. 2010; 1127–1133.
- Sharma A, Sharma V, Kumar Kumawat T, Seth R. A Review on Antidermatophytic Efficiency of Plant Essential Oil. Int J Pure App Biosci. 2014; 265-278.
- Dias N, Dias MC, Cavaleiro C, Sousa MC, Lima N, Machado M. Oxygenated monoterpenes-rich volatile oils as potential antifungal agents for dermatophytes. Nat Prod Res. 2017 Feb;31(4):460-464. doi: 10.1080/14786419.2016.1195379. Epub 2016 Jun 16. PMID: 27309978.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
