International Journal of Pharmaceutical Sciences and Developmental Research
Edaravone Protects against Vascular Oxidative Damage Induced by AAPH in Chick Embryo
Xin Wan1,2, Meng-Xun Luo1,2, Chong Jie1,2, Tong Wu1,2, Gui-Yuan Yu1,2, Yi-Fang Li1,2, Rong-Rong He1,2 and Hiroshi Kurihara1,2*
2Institute of Traditional Chinese Medicine & Natural Products, Jinan University, Guangzhou 510632, China
Cite this as
Wan X, Luo MX, Jie C, Wu T, Yu GY, et al. (2016) Edaravone Protects against Vascular Oxidative Damage Induced by AAPH in Chick Embryo. Int J Pharm Sci Dev Res 2(1): 019-022. DOI: 10.17352/ijpsdr.000007Background: Edaravone (Eda) is a free-radical scavenger which is used in treating stroke, cerebral hemorrhage and some other diseases in clinic. However, it’s antioxygenation during development is still unknown.
Method: An oxidative damage model was established in chick embryo by using a generator of free radicals, 2,2’-azobis[2-methylpropionamidine] dihydrochloride (AAPH). The mortality rate and embryo’s weight were measured to detect whether retarded growth happened. In order to observe the blood vessels, the yolk-sac blood vessels and CAM blood vessels density were observed. The malondialdehyde (MDA) content and superoxide dismutase (SOD) enzymatic activity were detected to evaluate the oxidative damage in the chick embryo.
Result: In this model, embryo development and angiogenesis were heavily affected by AAPH. However, Eda alleviated the growth retardation and anti-angiogenesis induced by AAPH. After AAPH treatment, the MDA content increased and SOD enzymatic activity declined which was also mitigated by Eda.
Conclusion: Overall, our research revealed that Eda mitigated embryonic anti-angiogenesis induced by oxidative damage after AAPH treatment, which may be helpful for future clinical studies.
Abbreviations
Eda: Edaravone; 3-methyl-1-phenyl-2-pyrazolin-5-one; AAPH: 2,2’-azobis[2-methylpropionamidine] dihydrochloride; EDD: Embryo Development Day; MDA: Malondialdehyde; SOD: Superoxide Dismutase; CAMs: Chorioallantoic Membranes; IPP: Intel Integrated Performance Primitives; TBA: Thiobarbituric Acid
Introduction
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one, Eda), a free-radical scavenger, is widely used in treating stroke, cerebral hemorrhage and some other diseases in clinic [1]. Its pharmacological effects on cardiovascular diseases and brain infarct have been confirmed by numerous in vivo experiments [2-4]. Previous research showed that Eda exerted an excellent antioxygenation in chick embryos as it alleviated the oxidative stress by high glucose treatment [5]. However, whether it has an antioxygenation effect during embryo development is unclear. In order to observe the antioxygenation effect of edaravone during embryo development, we established a new chronic oxidative damage model by injecting AAPH to chick embryos in long-term.
Oxidative stress is a condition that an unbalance happens between the oxidative system and antioxidant system. It involves in many pathogenesis of diseases, including hypertension, cancer, diabetes and Alzheimer’s disease [6]. During development, excessive reactive oxide species do harm to the embryo. The cardiovascular system is the earliest formed functional system, which is sensitive to oxidative stress [7]. During the pregnancy period, pregnant women suffer from emotional fluctuation, environmental pollution, life pressure, improper diet and so on. Women are exposed to oxidative stress and the fetuses are easily affected.
In this research, the chick embryo was chosen as the experimental animal, as chick embryo is suitable for evaluating angiogenesis. The chick embryo holds a wide range of advantages, with low cost and less ethical arguments [8]. It is a classical development model where the chick yolk sac and chorioallantoic membranes (CAMs) are extensively used in research related to angiogenesis. Besides, the growth cycle of chick embryo is short, where 21 days for chick embryo is similar to 10 months for human [9]. Moreover, it is a simple spinal biological, whose growth process can be easily observed [10].
In order to mimic chronic oxidative stress exposed to human, 2,2’-azobis[2-methylpropionamidine] dihydrochloride (AAPH) was repeatedly treated in a small dose. AAPH is an azo compound and completely dissolved in alkyl, peroxyl and alkoxyl radicals at physiological temperature [11]. AAPH has been widely used to study the mechanism of oxidative stress [12,13]. In our previous experiments, the chick embryo on embryo development day (EDD) 9 was treated with a large dose of AAPH. After incubation for 24 hours, the chick embryo suffered from oxidative stress and the cardiovascular system was affected [7]. Nevertheless, whether chronic oxidative stress affects the development of embryos is still unclear. In our study, AAPH was injected to chick embryos in long-term and the development of chick embryo was observed.
Materials and Methods
Animals and chemicals
Fertilized Leghorn eggs were provided by the Avian Farm of the South China Agriculture University (Guangzhou, China). AAPH was bought from Sigama-Aldrich (Saint Louis, Mo, USA). Eda was the product of Sigama-Aldrich (Saint Louis, Mo, USA). Sodium chloride, potassium chloride, sodium dihydrogen phosphate and monopotassium phosphate were analytically pure and were purchased from Aladdin Chemicals (Shanghai, China).
Experimental groups
Chick embryos were incubated in an egg’s incubator (Grumbach, Wetzlar, Germany), at a temperature of 38 °C and a humidity of 70-75%. On EDD 3, different concentrations of AAPH (0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00 µmol/egg) were injected to chick embryos. In the control group, 0.72% sodium chloride was injected into the albumen. On EDD 5, EDD 7 and EDD 9, chick embryos were treated as the same as EDD 3.
In the following experiments, 0.50 µmol/egg was chosen as the dosage of AAPH. The chick embryos were randomly divided into 3 groups, control group (0.72% sodium chloride), AAPH group and AAPH plus Eda group, respectively. The number of embryos in every group was more than 15. On EDD 0, the group of AAPH plus Eda group was treated with Eda at a concentration of 1 nmol/egg. The AAPH group and AAPH plus Eda group were treated with AAPH at a concentration of 0.50 µmol/egg on EDD 3, EDD 5, EDD 7 and EDD 9. The control group was treated with isopyknic 0.72% sodium chloride.
Blood vessels density of CAMs and yolk-sac in chick embryo
The shell of the eggs was removed and the chick embryo was collected in a petri dish on EDD 6. At this moment, the embryos and blood vessels were on the upper of the yolk, where it was easy to observe the embryos and the blood vessels. Then the morphology of the blood vessels was photographed. On EDD 10 and EDD 16, the blunt end of eggs was taken down and the CAMs were adhered to the egg shells. The morphology of the CAMs was photographed. The areas occupied by the blood vessel were measured by Intel Integrated Performance Primitives (IPP) [14]. The blood vessel density was the percentage of area occupied by the blood vessels over the whole area under the picture’s field [15].
The measurement of MDA content and SOD activity
The chick embryos were collected on EDD 10. Malondialdehyde (MDA) content and superoxide dismutase enzymatic (SOD) activity were detected by kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. MDA contents were determined according to thiobarbituric acid (TBA) method. The assay is based on the reaction of MDA with TBA, forming an MDA-TBA adduct that is absorbed strongly at 532 nm. The activity of SOD was detected by xanthine oxidase technique. NBT is a reagent that absorbs superoxide and its color turns into purple. The SOD enzymatic activity can be measured by absorbance value (OD 450).
Statistical analysis
All data were shown as mean ± SD. One-way ANOVA was tested for differences among experimental and control groups for multiple comparisons. Significance was determined by p-values less than 0.05.
Results
AAPH induced chick embryo growth retardation
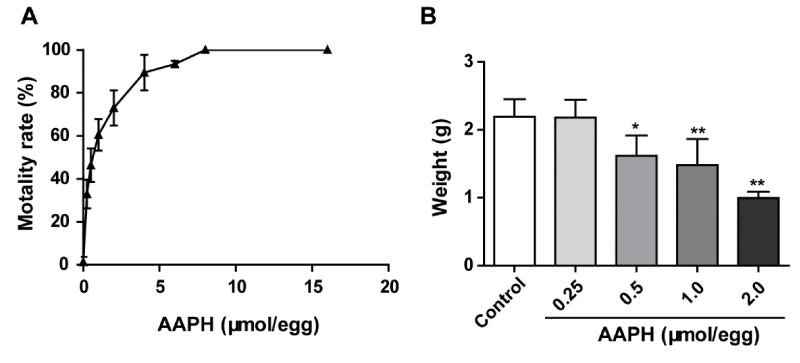
In order to examine the influence of AAPH on the development of chick embryo, different dosages of AAPH (0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00 µmol/egg) were treated to embryo on EDD 3 once every two days. The chick embryos were sacrificed on EDD 10 and the mortality increased after AAPH treatment. The LD50 was 0.59 µmol/egg and the chick embryos died thoroughly when the dosage was above 8.00 µmol/egg (Figure 1A). Meanwhile, when the dosages of AAPH were more than 0.50 µmol/egg, the weight of the whole embryo declined obviously (Figure 1B), which indicated that AAPH caused retarded growth. Therefore, 0.50 µmol/egg was selected as the dosage of AAPH in the following experiments.
Eda improves the retarded growth caused by AAPH
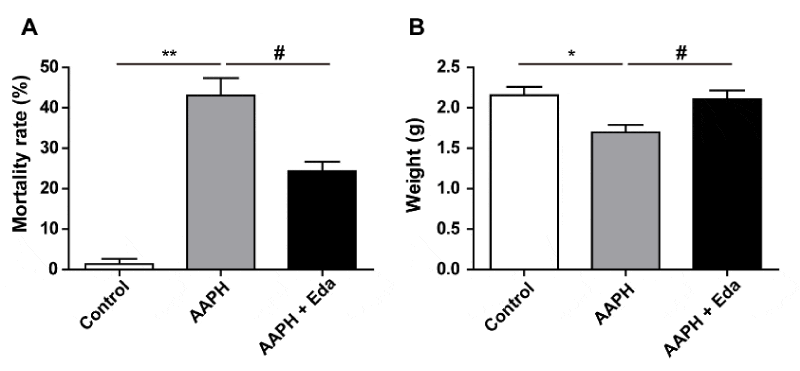
When the chick embryo was exposed to AAPH at a concentration of 0.50 µmol/egg, the mortality was 43.00 ± 7.55%. However, the mortality of the embryos in Eda plus AAPH group was 24.33 ± 4.04% (Figure 2A), suggesting that Eda improved the survival rate. Meanwhile, Eda treatment significantly inhibited the decline in the weight of chick embryo induced by AAPH treatment (Figure 2B). Above results showed that Eda mitigated the retarded growth caused by AAPH treatment.
Eda remits the damage of yolk-sac blood vessels induced by AAPH
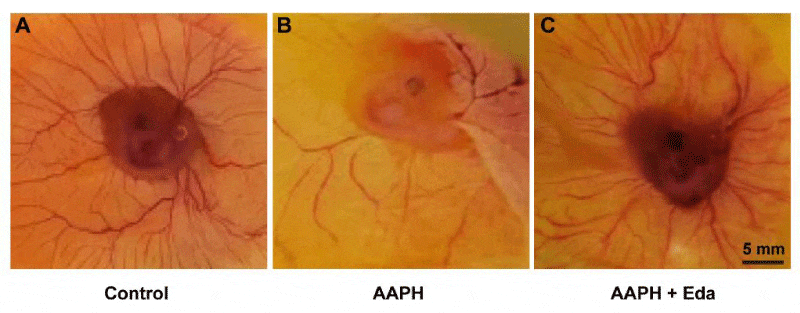
Chick embryo was put in a petri dish on EDD 6 and the morphology of the blood vessels was photographed. As shown in Figure 3, AAPH exposure group had reduced the density of blood vessels on yolk-sac (Figure 3A, B) and Eda obviously reduced the damage caused by AAPH treatment (Figure 3C). These images indicated that Eda had a protection against AAPH-impaired angiogenesis in the yolk sac of the chick embryo.
Eda improves the damage of CAM blood vessels induced by AAPH
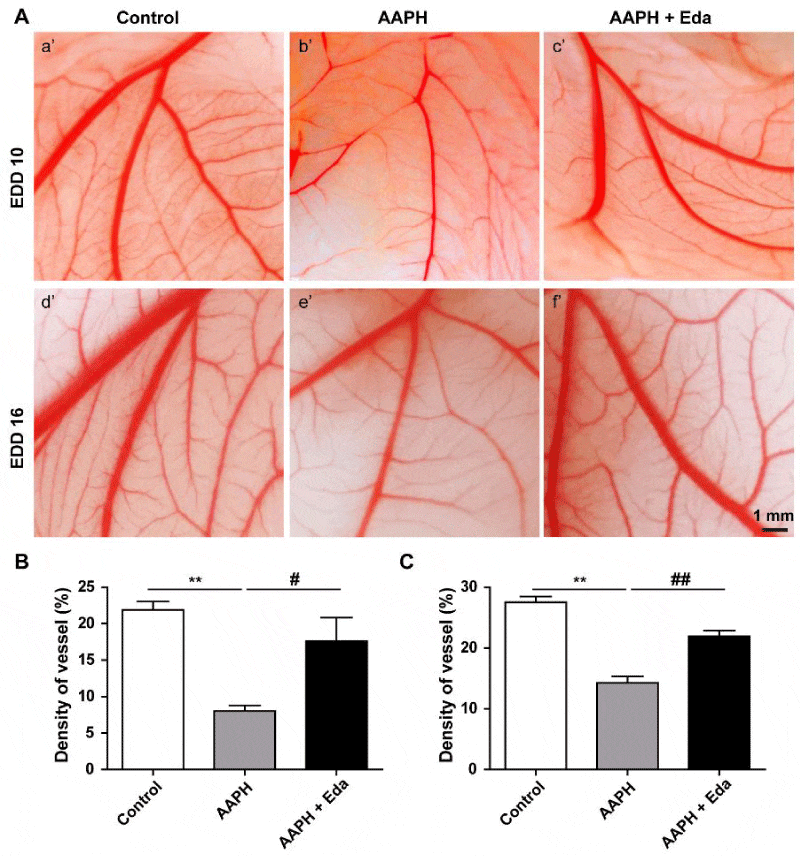
On EDD 10 and EDD 16, the CAMs of chick embryo were observed. As shown in Figure 4A, the primary, secondary, tertiary vessels and their branches were clear in the control group on EDD 10 (a’ in Figure 4A) and EDD 16 (d’ in Figure 4A). However, the blood vessels in the embryos of AAPH group were discontinuous and unclear on EDD 10 (b’ in Figure 4A) and EDD 16 (e’ in Figure 4A). After Eda treatment, the blood vessels were more clear, compared to the blood vessels on EDD 10 (c’ in Figure 4A) and EDD 16 (f’ in Figure 4A) after AAPH treatment. The blood vessels density was 21.89 ± 1.97% in control group. The density dropped to 8.04 ± 1.31% after AAPH treatment, while it was elevated to 17.64 ± 5.55% by Eda (Figure 4B) on EDD 10. In parallel, the density also sharply declined in AAPH group compared with control group (14.26 ± 2.17% vs. 27.56 ± 1.78) on EDD 16, which was significantly reversed by Eda treatment (21.92 ± 1.88%, Figure 4C).
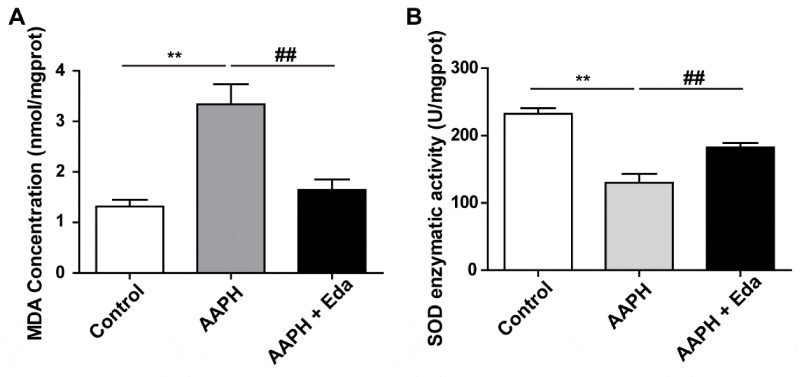
Eda inhibited AAPH-induced oxidative damage
The whole embryo on EDD 10 was harvested to determine the content of MDA and the enzymatic activity of SOD. MDA was a typical substance which reflected the degree of lipid peroxidation. Meanwhile, SOD was a representative enzyme of antioxidant system. After AAPH treatment, the MDA content of the chick embryo was dramatically elevated, while Eda significantly decreased MDA content comparing with AAPH group (Figure 5A). In the meantime, AAPH treatment significantly reduced SOD enzymatic activity, which could be suppressed by was Eda treatment (Figure 5B).
Discussion
Increased ROS production in vascular has been related to various cardiovascular diseases, including hypertension [16]. Many reports show that immoderate ROS involves in the formation of vascular diseases [17,18]. However, few reports figured out whether excess ROS in the placenta influenced the cardiovascular system of the embryo. In our study, AAPH was used to establish a chronic oxidative damage model in chick embryo to mimic oxidative damage in the human embryo. There are two sets of the extraembryonic circulation system in the chick embryo, the CAM vascular circulation system and the yolk sac circulation system. The CAM is widely used in studying angiogenesis, drugs delivery, toxicologic analysis, tissue grafts, tumor growth, metastasis and so on [19]. CAM is important in the development of chick embryo, which carries oxygen as well as nutrients to chick embryos. During the entire phase of embryogenesis, the nutrient is supported by the yolk sac of the avian embryo for its development, as the placenta in mammals [20].
In our previous published study, the chick embryo on EDD 9 was treated with AAPH. The embryos were obtained on EDD 10 and the LD50 of AAPH treatment was 10.00 µmol/egg [7]. In the present study, the LD50 of AAPH treatment was 0.5 µmol/egg. The reasons accounting for this difference may be due to distinct treatment time points and frequency. The embryos were more sensitive to stimulations on early stage, so that the dose of AAPH in our previous research was much higher. In our study, AAPH caused growth retardation, reflected by the reduced weight of chick embryo. The employment of antioxidant Eda obviously mitigated this condition. Meanwhile, AAPH reduced the blood vessels density of the yolk sac. Apart from yolk sac, the blood vessels density of the CAM dramatically decreased after the exposure of AAPH. Promisingly, Eda treatment prevented the reduction of blood vessels density on CAM and yolk sac. The influence of AAPH on embryo growth probably was related to the abnormal angiogenesis in the chick embryo, which still needs more evidences in the future.
Further, we found that Eda administration prevented the AAPH-induced increase of MDA content and decreased of SOD activity in the chick embryo. It can be inferred that Eda exerted an extraordinary effects on protecting angiogenesis and embryo growth was through its anti-oxidative effects. As chick embryo is more similar to living animals with little ethical arguments, it is an ideal model for evaluating the effects of antioxidants on oxidative damage-induced angiogenesis and growth retardation.
Conclusion
In this model, embryo development and angiogenesis were heavily affected by AAPH.
The yolk-sac blood vessels density dramatically declined on EDD 6 and CAM blood vessels density also decreased on EDD 10 or EDD 16. Eda alleviated the growth retardation and anti-angiogenesis induced by AAPH. After AAPH treatment, the MDA content increased and SOD enzymatic activity declined which was mitigated by Eda. Overall, our research revealed that AAPH induced embryonic anti-angiogenesis through oxidative damage and Eda alleviated the damage, which may be helpful for future clinical studies.
- Ren YX, Wei B, Song XR, An N, Zhou YY, et al. (2015) Edaravone's free radical scavenging mechanisms of neuroprotection against cerebral ischemia: review of the literature. Int J Neurosci 125: 555-565 .
- Tsujita K, Shimomura H, Kawano H, Hokamaki J, Fukuda M, et al. (2004) Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 94: 481-484 .
- Otomo E, Tohgi H, Kogure K, Hirai S, Takakura K, et al. (2003) Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction-Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis 15: 222-229.
- Shichinohe H, Kuroda S, Yasuda H, Ishikawa T, Iwai M, et al. (2004) Neuroprotective effects of the free radical scavenger Edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res 1029: 200-206 .
- Tan RR, Zhang SJ, Li YF, Tsoi B, Huang WS, et al. (2015) Proanthocyanidins Prevent High Glucose-Induced Eye Malformation by Restoring Pax6 Expression in Chick Embryo. Nutrients 7: 6567-6581 .
- Kreuz S, Fischle W (2016) Oxidative stress signaling to chromatin in health and disease. Epigenomics 8: 843-862 .
- He RR, Li Y, Li XD, Yi RN, Wang XY, et al. (2013) A New Oxidative Stress Model, 2,2-Azobis(2-Amidinopropane) Dihydrochloride Induces Cardiovascular Damages in Chicken Embryo. PLoS One 8: e57732 .
- Vargas A, Zeisser-Labouebe M, Lange N, Gurny R, Delie F (2007) The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv Drug Deliv Rev 59: 1162-1176.
- Martinsen BJ (2005) Reference guide to the stages of chick heart embryology. Dev Dyn 233: 1217-1237 .
- Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88: 49-92.
- Nakajima A, Yamaguchi T, Yamashita T, Kawai K, Miyake Y, et al. (2015) Quantitative Spin-trapping ESR Investigation of Alkoxyl Radical Derived from AAPH: Development of a Flow-injection Spin-trapping ESR System for the Oxygen Radical Absorbance Capacity Assay. Appl Magn Reson 46: 1013-1022 .
- Duan WJ, Li YF, Liu FL, Deng J, Wu YP, et al. (2016) A SIRT3/AMPK/autophagy network orchestrates the protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 264.7 macrophages. Free Radic Biol Med 95: 230-242 .
- He RR, Yao XS, Li HY, Dai Y, Duan YH, et al. (2009) The Anti-stress Effects of Sarcandra glabra Extract on Restraint-Evoked Immunocompromise. Chem Pharm Bull 32: 247-252 .
- Wang G, Zhong S, Zhang SY, Ma ZL, Chen JL, et al. (2016) Angiogenesis is repressed by ethanol exposure during chick embryonic development. J Appl Toxicol 36:692-701 .
- He YQ, Li Y, Wang XY, He XD, Jun L, et al. (2014) Dimethyl phenyl piperazine iodide (DMPP) induces glioma regression by inhibiting angiogenesis. Exp Cell Res 320: 354-64 .
- Touyz RM, Briones AM (2011) Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 34: 5-14.
- Weseler AR, Bast A (2010) Oxidative Stress and Vascular Function: Implications for Pharmacologic Treatments. Curr Hypertens Rep 12: 154-161 .
- Sarre A, Pedretti S, Gardier S, Raddatz E (2010) Specific inhibition of HCN channels slows rhythm differently in atria, ventricle and outflow tract and stabilizes conduction in the anoxic-reoxygenated embryonic heart model. Pharmacol Res 61: 85-91 .
- Ribatti D (2016) The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech Dev 141: 70-77 .
- Yadgary L, Wong EA, Uni Z (2014) Temporal transcriptome analysis of the chicken embryo yolk sac. BMC Genomics 15: 690 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
