International Journal of Pharmaceutical Sciences and Developmental Research
Antioxidant Properties of Rhazya stricta Decne
1Department of Chemistry, Mirpur University of Science and Technology (MUST), New Campus, Mirpur-10250 (AJK), Pakistan
2Department of Biotechnology, Mirpur University of Science and Technology (MUST), City Campus, Mirpur-10250 (AJK), Pakistan
Author and article information
Cite this as
Habib-ur-Rehman, Asghar R. Antioxidant Properties of Rhazya stricta Decne. Int J Pharm Sci Dev Res. 2025;11(1):006-014. Available from: 10.17352/ijpsdr.000056
Copyright License
© 2025 Habib-ur-Rehman, er al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Abstract
Rhazya stricta Decne is an evergreen dwarf shrub belonging to the Apocynaceae family. The plant is widely distributed in the Middle East and the Indian sub-continent. The plant has a long history of use in folk medicine for the treatment of several diseases, including cancer. The extracts of the plant are prescribed for the treatment of various disorders such as diabetes, sore throat, helminthiasis, inflammatory conditions, fever, and chronic rheumatism in the areas where it grows. The extracts of plants possess radical scavenging activities and have shown antioxidant activities in a variety of bioassays, including animal models. The plant-extract-based nanoparticles also exhibited antioxidant activity. A detailed account of the antioxidant activity of the extracts of the plant is presented.
Introduction
The increased life expectancy in the present century and a significant increase in the aging population pose a huge challenge to maintain a healthy old age [1]. Compelling evidence implicates the causal role of reactive oxygen species, free radicals, oxidative and nitro-oxidative stress in the metabolic imbalances, progressive tissue damage, and early aging [2,3]. In view of the complexity of the aging process and associated degenerative processes, comprehensive solutions are being searched from complementary and alternative medicine to provide a holistic approach to reverse, arrest, delay, or repair the progressive deterioration of the aging cells. Research is being carried out to find out holistic and herbal solutions from the Ayurveda system of medicine for preventing oxidative stress-linked tissue damage and providing significant antioxidant defenses to promote longevity and rejuvenation [3].
The Ayurvedic Rasayana herbs have been used in the Indian system of medicine for centuries to revitalize and rejuvenate the whole functional dynamics of the body system. The Rasayana herbs exert multi-dimensional health benefits and generally possess strong antioxidant activity, though only a few have been scientifically investigated. The common Rasayana herbs that are used in practice have been investigated. Some of the molecular targets of commonly used herbal antioxidants and rejuvenators were identified. The attempts are an effort to share insights on the concepts behind the application of herbal antioxidants to promote longevity in the light of the scientific underlying molecular mechanisms [3].
The antioxidants protect against infection and degenerative diseases by neutralizing the effects of the free radicals. The synthetic antioxidants, including butylated hydroxy anisole (BHA), butylated hydroxyl toluene (BHT), tertiary butylated hydroquinone, and gallic acid esters, inhibit the oxidation processes in the body. They may serve as chelating agents, such as ethylene diamine tetraacetic acid (EDTA), and can bind metals to reduce their contribution in the process [4]. They are thought to cause or promote negative health effects such as mutagenesis and carcinogenesis in humans [5]. Therefore, there is a strong trend to replace the synthetic antioxidants with naturally occurring ones to prevent free radical-related diseases without any negative health effects [6].
The natural antioxidants help in controlling the formation of free radicals and activated oxygen species, or can inhibit their reaction with biological structures [7]. These antioxidants include anti-oxidative enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase, and small non-enzymatic antioxidant molecules, such as glutathione and vitamins C and E [8]. Many herbs and spices, and plant extracts contain the chemical constituents with antioxidant properties [9-11]. The extracts of Rhazya stricta also contain radical scavenging properties and have shown excellent antioxidant activities.
Rhazya stricta Decne is widely distributed in the Middle East and Western Asia and is abundantly found in all regions of Pakistan [12-14]. The plant has long been used in the indigenous system of medicine for the treatment of various ailments, including cancer [15]. The extracts of the plant have shown a wide spectrum of biological activities, including antioxidant activities. Because of increased safety concerns about synthetic antioxidants, the exploitation of cheaper and safer sources of antioxidants based on natural origin is the focus of the research being carried out nowadays. The plant-extract-based nanoparticles have also been synthesized and characterized [16]. The ethanol extract of the plant-based AgNPs has shown better antioxidant activity than the crude ethanol extract.
Results and discussion
Because of the increased safety concerns about various synthetic drugs and supplements, including antioxidants, many efforts are being carried out to explore cheaper and safer sources of antioxidants based on natural sources. The present-day researches are focused on finding natural products with antioxidant properties. Rhazya stricta is one of the medicinally important plants known for its medicinal properties, including antioxidant activities. The extracts of the plant contain several chemical constituents, including antioxidants [17]. The experiments showed that the methanol extract of the plant exhibited the highest total phenolic content; it was selected for further studies. Antioxidant activity was measured in the linoleic acid system, metal chelating activity, reducing power, scavenging effect on 1, 1-diphenyl-2-picrylhydrazyl free radical, and superoxide anion radical scavenging activity were considered as parameters for the assessment of the antioxidant potential of the methanol extracts of the plant. The results were compared with α-tocopherol and the synthetic antioxidant butylated hydroxyanisole. The antioxidant potential of the methanol extracts of the leaves of the plant was comparable with the previously exploited potent antioxidants, and the results have shown that the activity of the extracts is strongly dose dependent.
Antioxidant Activity Spectrum of Rhazya stricta
The in vitro antioxidant activity of the extract of Rhazya stricta was determined. The leaves of the plant were extracted with 70% ethanol. The extract was subjected to the DPPH (1, 1-diphenyl-2-picrylhydrazyl) assay to determine its antioxidant activity. The result showed that the extract possesses antioxidant activity [18]. In addition to the antioxidant activity, the extract also showed anti-proliferative and anti-metastatic activities at low concentrations, and the anti-proliferative activity of the extract was found to be consistent with its antioxidant activity.
Rhazya stricta has been shown to have an antioxidant action in rats [19]. The methanol extract of the leaves of the plant exhibited the highest total phenolic content and an antioxidant potential that was comparable with the previously exploited potent antioxidants of the plant [20]. The methanol extracts of the leaves of the plant showed the presence of a high amount of phenolic content and antioxidant potential. The photochemistry, bioactivities, and ethnomedicinal uses of the plant were reviewed [21]. The ethanol extract of the fruit of the plant has shown significant lipoxygenase and acetylcholine esterase activities [22]. The plant extracts have also shown good antioxidant activity in the animal models of the diseases [23]. The extract of the leaves has shown a prominent dose-dependent effect on the reduced glutathione, lipid peroxidation, and ascorbic acid concentrations in the liver and kidneys of rats. It was concluded that at some of the doses used, the extract has antioxidant activities [24].
The extracts of the plant have also shown antioxidant activities in animal models. At some doses, the extract exhibits antioxidant activity in rats by increasing the glutathione levels and decreasing the lipid peroxidation [24]. In comparison to the tocopherol drug and the synthetic antioxidant butylated hydroxyanisole, the methanol extract of the plant was a significant source of the natural antioxidants with strong free radical scavenging and anion radical scavenging potentials [20]. The significant lipoxygenase and acetylcholinesterase inhibitory activity was observed by the ethanol extract of the fruit of the plant [22].
The antioxidant and immune responses of broiler chickens supplemented with the extract of Rhazya stricta in drinking water were determined [25]. The impact of the extract supplement on broiler chickens’ performance, especially the immune system, was investigated. The cellular immunity was evaluated by determining the phagocytic activity and lymphocyte proliferation. The humoral immunity was assessed by quantification of the serum total IgM and IgG. The serum levels of the antioxidant enzymes were measured, and a histological examination of the spleen and bursa of Fabricius was performed. The results showed a significant enhancement in the cellular immunity in the group supplemented with the water extract of the plant, as demonstrated by a significant increase in the phagocytic activity, lymphocyte proliferation, and percentages of circulating lymphocytes (p < 0.05). The chickens treated with the plant extract exhibited an enhanced humoral response, shown by a significant elevation in the serum levels of the total antibodies of the immunoglobulin M (IgM) and immunoglobulin G (IgG) isotypes, along with a notable increase in the bursa of Fabricius activity. Furthermore, plant supplementation is associated with a significant increase in the serum levels of catalase and superoxide dismutase (p < 0.05), along with a significant improvement in the broilers’ general performance, body weight, and feed regulation. The results suggested an immune-modulatory effect for the methanol extract of the plant and highlighted the potential use of the plant in preventive and therapeutic medicines.
The impact of the climate conditions of the plant on the activity was also determined. The plants collected from Riyadh and the western region of Saudi Arabia were subjected to the bioassay experiments to determine their antioxidant activity by six evaluations of superoxide radical scavenging and scavenging of hydrogen peroxide levels [26]. Both samples of the plant showed antioxidant activity, while the plant collected from the western region indicated a significantly superior performance. The antioxidant activity of the fractions obtained from the root of the plant was also determined by using a variety of antioxidant assays. The fractions obtained by solvent-solvent extraction of the crude methanol extract (CME) of the plant have shown a remarkable free radical scavenging activity with the IC50 value of 400 - 776 μg / ml [27 ].
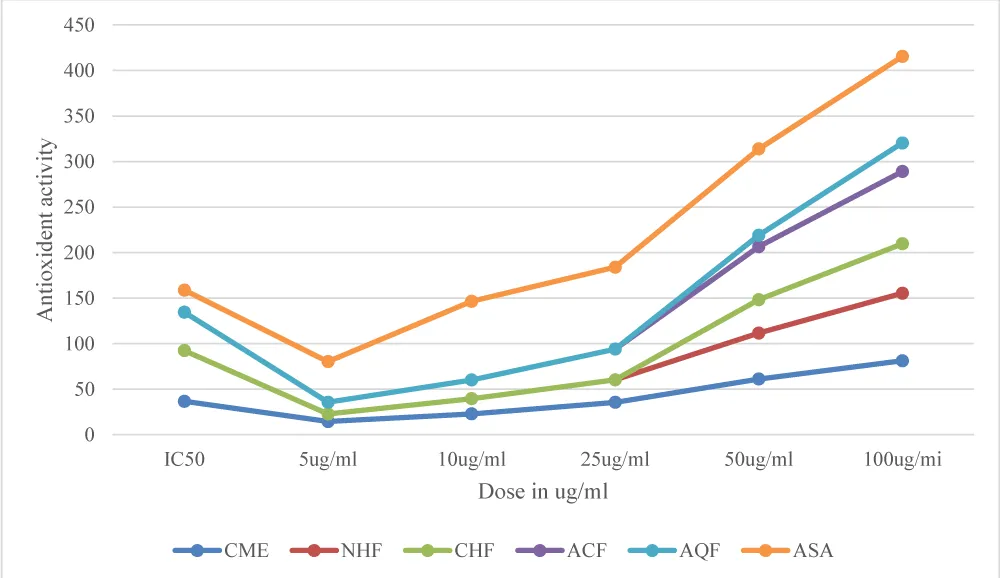
The antioxidant potential of the extract of the leaves of Rhazya stricta and its various fractions was analyzed by the DPPH free radical scavenging assay [28]. The crude methanol extract (CME) as well as its fractions showed effective free radical scavenging activity. The crude methanol extract has shown maximum antioxidant activity with the IC50 value of 36.59 µg/ ml. The acetone fraction (ACF) showed good antioxidant activity with an IC50 value of 42.15 µg/ ml, while the n-hexane fraction (NHF) showed an IC50 value of 55.7 µg/ ml. The chloroform fraction (CHF) and aqueous fractions (AQF) showed the lowest free radical scavenging activity with IC50 >100 µg/ ml. The crude methanol extract showed excellent free radical scavenging with IC50 36.59 µg/ ml, which is comparable to ascorbic acid (ASA). The phytochemical assay of the crude methanol extract showed that it has high concentrations of phenols, which are known to be potent antioxidants. The dose-response experiments showed a linear relationship between the dose strength and activity. The results of free radical scavenging assays are presented in Table 1.
The antioxidant activities of the crude methanol extract (CME), acetone fraction (ACF), n-Hexane fraction (NHF), chloroform fraction (CHF), aqueous fractions (AQF), and ascorbic acid (ASA) as a standard were compared with different doses to ascertain the dose response. The dose-response experiment showed that the extracts of the plant possess more antioxidant potential than the standard. The graphical presentations of antioxidant activities versus doses of different extracts of the plant are presented in Figure 1.
Total phenolic and flavonoid contents
The total phenolic contents of the ethanol extract of Rhazya stricta were determined by the Folin-Ciocalteau method using gallic acid as a standard [15]. The plant contains the highest total polyphenols (11.5 ± 0.01 mg gallic acid equivalent (GAE)/g of extract). A study showed that the total phenolic content of the extracts of the plant collected from different regions of Saudi Arabia ranged between 62.5 ± 0.2 and 66.63 ± 0.03 mg GAE /g of extract [26].
The total phenolic content of the extract and fractions of the plant was determined by using the Folin-Ciocalteu reagent and expressed in terms of gallic acid equivalents per gram of the extract [27]. The results showed that the highest level of phenolic content was found in the SCL fraction, while the crude extract of the roots showed the second highest amount of phenolic content. The FCL and TAL were found to have almost equal amounts of the contents, while the FAL contains the least phenolic content.
The total flavonoid content was estimated as milligram equivalents of quercetin per gram of extract at 415 by a colorimetric method, and a calibration curve was made with standard solutions of quercetin (0.1 to 25 μg/ ml). Interestingly, the highest flavonoid concentration was found in the SCL fraction rather than its parent fraction. Other fractions also contain a good level of flavonoids [27].
The phenolic contents of the plant were also determined by using the reported method [29]. The total phenolic content in relation to the gallic acid equivalent of the methanol extract of the plant was determined as 189.9 μg GAE /mg of extract [30]. The polyphenols, phenolic, and flavonoid contents of the ethanol extract of the plant are presented in Table 2
Polyphenol, phenolic, and flavonoid contents are taken as average, and calculated values are based on the experimental values; Gallic acid equivalent (GAE), Quercetin equivalent (QE)
The flavonoids and their derivatives have a wide range of biological activities, including anticancer activity. The anticancer activity of the flavonoids is attributed to their potent antioxidant effects, which include metal chelation and free-radical scavenging activities [31]. The flavonoids present in the herbs were found to contribute significantly towards their antioxidant properties [32]. The flavonoid content was obtained using the aluminium chloride assay, based on the formation of a complex between the aluminium ion and the carbonyl and hydroxyl groups of flavones and flavonols [33]. The flavonoid contents of the extract of the plant were found to be 9.2 ± 0.22 mg quercetin equivalent (QE) per gram. The relationship between the total flavonoid content and the free radical scavenging activity was studied using linear regression analysis. The results showed a positive correlation between the DPPH scavenging activity and the total flavonoid content. The comparison suggested that the other compounds may have also been participating in the radical scavenging activity of the plant extracts. The flavonoid content in the methanol extracts of the plant with respect to quercitin equivalent (QE) was found to be 27.7 μg QE/mg [29,30].
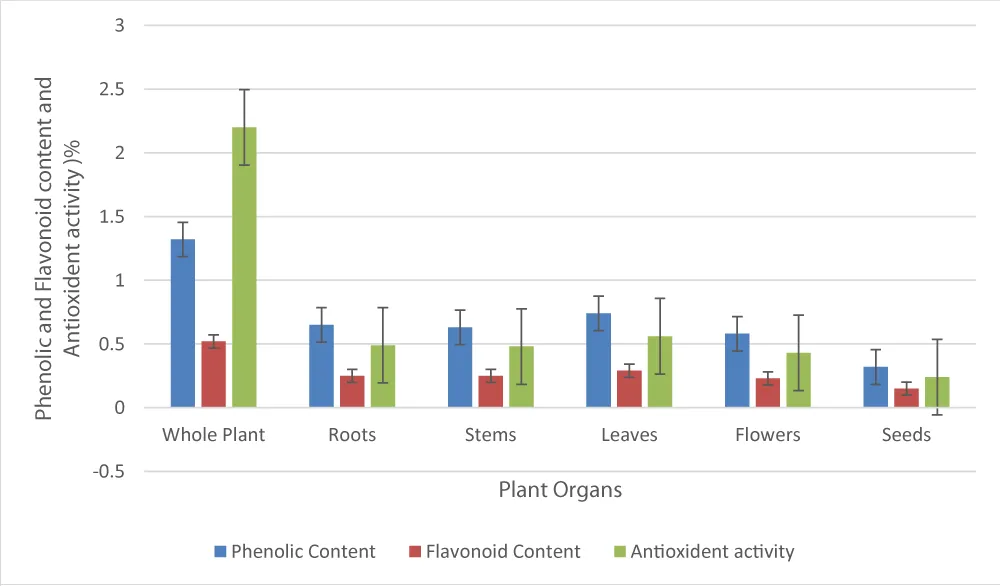
The phytochemical analysis and biological activity of different parts of Rhazya stricta showed the presence of significant amounts of phenolic and flavonoid compounds in the extracts of different parts of the plant [34]. The plant contains phenolic compounds ranging from 1.24% - 1.40% and flavonoids 0.30% - 0.74%. The highest concentration of the antioxidants was found in the leaves of the plant at 0.56%. The extracts of the roots, stems, leaves, flowers, and seeds contain 0.49, 0.48, 0.56, 0.43, and 0.24 grams of antioxidant content per 100 g of the plant, respectively. The proportion of phenolic and flavonoids and antioxidants in the whole plant and its different parts is presented in Table 3.
The data revealed that the phenolic and flavonoid compounds contribute to the antioxidant properties of the plant. The result showed that the phenolic and flavonoid contents of different parts of the plant correspond to the antioxidant activity of the extracts. The proportion of phenolic and flavonoid contents of the whole plant and its different parts, with their antioxidant activities, is presented in Figure 2.
The antioxidant and free radical scavenging activities of Rhazya stricta collected from different regions of Saudi Arabia were evaluated [26]. The extracts of the plant were subjected to screening for their possible antioxidant properties. The four complementary test systems, namely superoxide radical scavenging assay, scavenging of hydrogen peroxide, total phenolic compounds, and total flavonoids, were used for the purpose.
The scavenging percentage of hydrogen peroxide was varied significantly within the plant types as well as different habitats of the plant, for instance, R. stricta collected from Riyadh region has shown only 35.7% scavenging activity. It was found that the change in the plant habitats, ranging from the middle region (Riyadh) to the West, enhanced the degree of the scavenging of hydrogen peroxide from 35.7% to 48.3%. The plants responded to some unfavorable conditions by activating the antioxidant defense system, including the enzymatic and non-enzymatic chemical constituents. The results suggested that the higher percentage of the scavenging of superoxide radicals, as well as the scavenging of hydrogen peroxide, indicated the higher activities of the antioxidant defense system in the plants to face the presence of some adverse conditions.
Similarly, the results showed that the superoxide radical scavenging assay was varied significantly in the plants collected from Riyadh and the western region. The corresponding values for the plant collected from the Riyadh region were 43.2% and it enhanced to 57.4% for the plants collected from the western regions, respectively. As described previously, the antioxidant activity of the extracts was assessed based on their ability to scavenge the stable 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical [35]. The antioxidant potential of the plant was determined by using a free radical scavenging assay [30]. The methanol extract of the plant was subjected to the free radical scavenging assay by using the reported method [36]. The methanol extract exhibited 38.39% DPPH scavenging activity.
The extract of the leaves of the plant was also evaluated for its antioxidant activity in vitro through the ABTS radical scavenging assays, and the IC50 values were determined [37]. The extract exhibited significant antioxidant properties, with the inhibition rates varying from 92.41% to 22.76% across concentrations of 500 to 15.625 μg/ ml. The IC50 value was determined to be 45.36 μg/ ml. The radical scavenging activity of the aqueous methanol extract of the leaves of the plant was assessed using the ABTS scavenging assay. The extract was able to get rid of radicals with an IC50 value of 45.36 μg/ ml and inhibition rates ranging from 92.41% to 22.76% at 500 to 15.62 μg/ ml. The 2,2’-azinobis (3-ethylbenzothiazoline-6 sulfonic acid (ABTS) scavenging assay showed statistically significant changes with the different concentrations of the extract of the plant. The study showed that the extract was more effective in stopping free radicals at the highest levels. The extract showed the strong ABTS inhibition and hence the ABTS free radical scavenging activity. The data indicated that various fractions of the extracts of the plant may act as a natural antioxidant source [38].
The DPPH Free Radical Scavenging Activity DPPH assay was used to measure free radical scavenging activity of the plant extract and its fractions [27]. All fractions were analyzed at a 1 mg/ ml concentration. All fractions were reanalyzed at lower concentrations for the determination of the IC50 of all the fractions. The IC50 values ranged from 313.5 μg/ ml to 776.1 μg/ml. The crude extract of the roots of the plant has shown good scavenging activity, while the SCL fraction exhibited the second most potent fraction after the crude extract of roots. The overall results showed the potential of the roots of the plant as a free radical scavenger. The results of the dose-response experiments are presented in Table 4.
The total antioxidant capacity of the extract of the roots of the plant and fractions was determined by the phosphomolybdenum method, pectrophotometrically. The highest total activity was observed by the SCL fraction, while the CER also exhibited remarkable activity.
CER: Crude extract of roots; FAL: First aqueous layer; SCL: Second chloroform layer; TAL: Third aqueous layer; FCL: Fourth chloroform layer; Standard: Ascorbic acid
As the reducing power ability is also an indicator of a plant’s antioxidant capacity, the extract of the roots and fractions were subjected to the total reducing power assay. The results showed good reducing power capacity of all fractions with reference to ascorbic acid equivalents per gram of extract. The SCL and FCL have shown good results as compared to the aqueous layers of the crude extract of the roots, while the RCE has shown maximum reducing power, followed by the second chloroform layer.
The antioxidant activity of the plant was also assessed based on its ability to reduce the stable DPPH radical [35]. The DPPH radical is stable and can accept an electron or a hydrogen radical and form a stable diamagnetic molecule by producing a color change from blue to yellow [39]. The color change of the DPPH has been widely used to measure the radical scavenging activity because of its stability, simplicity, and reproducibility [40]. The free radical scavenging capacity of the ethanol extracts of the plants was assayed based on the remaining amount of the DPPH radical percentage as a function of time. The total antioxidant activity was expressed as ascorbic acid equivalent per gram of the dry extract.
The percentage inhibition of the DPPH scavenging activity of the extract was dose-dependent. The DPPH scavenging activity of the plant was found to be 89.9% ± 0.51 at 1.5 mg/ml and 28.7% ± 1.27 at 0.15 mg/ ml. The results showed that there is no clear difference in the DPPH scavenging activities of the extract. The results are almost in agreement with their total polyphenol contents. Therefore, it may be concluded that the antioxidant activity of the plant might be related to its total phenolic content.
In addition, the DPPH radical scavenging potential of the extract was evaluated on the basis of the EC50 (μg/ ml) value. The extract of the plant contains the highest amount of total polyphenol content (11.5 μg/ ml) and has shown the highest scavenging activity (EC50 = 241.8 μg/ ml). The relationship between the total phenol content and the free radical scavenging activity based on the EC50 value was also studied using linear regression analysis. The results showed a significant negative correlation between the DPPH radical scavenging through the EC50 value and the total phenolic content. The correlation suggested that the presence of the phenolic compounds has contributed significantly to the antioxidant activity of the plant. These results are consistent with the previous works that showed a linear correlation between the total phenolic content and the reducing antioxidant capacity of some of the plant extracts [41].
The results clearly indicate that the methanol extract of the leaves of the plant exhibited good antioxidant activities with 92.41% inhibition at 500 μg/ ml. The results are similar to the previous studies, which showed that the difference in the rate of inhibition is due to the difference in the concentration of some of the active chemical constituents. Several reports have revealed that different parts of the plant possess different ABTS scavenging activity. The assessment of the antioxidant potential of different fractions of the extracts of the root of the plant was carried out by using different antioxidant assays. The fractions obtained through solvent-solvent extraction of the crude extract of the roots of the plant exhibited a significant (p < 0.001) free radical scavenging activity with the IC50 value ranging from 400 to 776 μg/ ml [27].
The phytocchemical, antioxidant, antimicrobial, and antibiofilm properties of the methanol extract of Rhazya stricta were studied [42]. The results showed that the methanol extract of the plant showed significant antioxidant activity in the free radical scavenging assay with an IC50 value of 74.2 μg/ ml. The extract also showed high superoxide dismutase (SOD) and peroxidase activities, which indicated an elevated antioxidant defense system in the plant.
The antioxidant potential of the plant was also determined by using the phosphomolybdenum method [43]. The methanol extracts of the plant were incubated in DMSO (100 μl of 4mg/ml DMSO), having reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate) and 4 mM ammonium molybdate for 90 minutes at 90 °C. Absorbance of this mixture (reaction mixture) was evaluated at 645 nm spectrophotometrically. Comparison was made with ascorbic acid (positive control) and a reagent having no sample (negative control), and the results were taken as an average of triplicate experiments [30].
The results of the antioxidant assays were supported by the previous reports in which the extracts of the plant were examined for their antioxidant potential, and the results showed that the extract has been shown to have significant antioxidant activity [44]. The fractions obtained by the solvent-solvent extraction of the raw extract of the roots of the plant have exhibited remarkable free radical scavenging activity with an IC50 value of 400 - 776 μg/ ml [27]. The direct comparison of the data from the current study with those reported in the literature is difficult since different parts and expression units were used. The IC50 values were determined based on the graph representing the concentration of the sample necessary to scavenge 50% of the ABTS. The IC50 is commonly utilized to indicate the concentration of extracts required to neutralize 50% of the free radicals.
The plant extract was subjected to the total reducing power assay [30]. The assay was carried out by using the reported procedure with minor modifications [45]. Ascorbic acid was employed as a standard. A mixture of 100 μl sample, potassium ferricyanide (1%), and phosphate buffer pH 6.6 (0.2 ml) was incubated at 50 ⁰C. The 10% trichloroacetic acid was mixed with the above mixture, and the resultant mixture was centrifuged for about 10 minutes at 3000 rpm. The reaction mixture supernatant was transferred to the 96-well plate, followed by the addition of distilled water and ferric cyanide 0.1%. The phosphate buffer was used as a blank. The absorbance values of the reaction mixture, standard, and blank were found at 760 nm, and the results were shown as µg of ascorbic acid that was equivalent per mg of extract after triplicate analysis.
Plant-based nanoparticles
The plant-based nanoparticles often show more activity than the traditional plant extracts due to their enhanced delivery, increased efficacy, and better penetration properties [46-48]. As the plant-based nanoparticles can offer improved therapeutic outcomes, the biologically active plant-based nanoparticles may be used to combat diseases, once they are found to be more effective and safe. The above discussion proved that the extracts of the plant have shown remarkable activities in a variety of bioassays. Keeping in view the properties of the nanoparticles and the activity spectrum of Rhazya stricta, it is very likely that the activities of the plant-based nanoparticles will be enhanced considerably. The plant extract-based nanoparticles have also shown promising biological activities and hence proved to be a potential source of therapeutic agents [16].
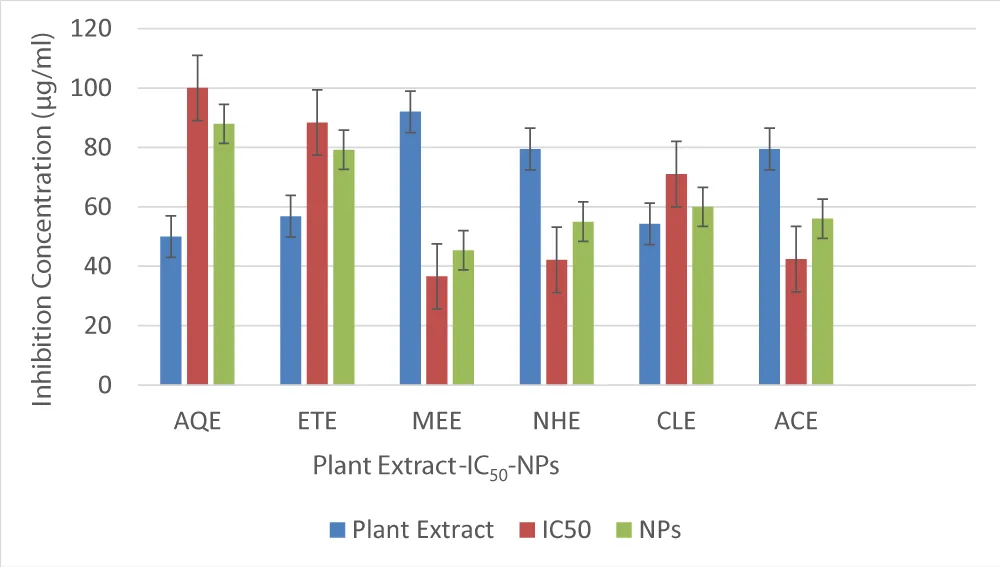
The AgNPs were synthesized by using aqueous and ethanol extracts of Rhazya stricta, and the synthesized nanoparticles were characterized with the help of UV-visible, Fourier transform infrared (FT-IR), and atomic force microscopy (AFM) methods. The thermal stability of the nanoparticles demonstrated that by increasing the reduction time and temperature, the absorption of AgNPs also increased, leading to more stable NPs formation. The plant-based nanoparticles were subjected to the DPPH assay to determine their antioxidant potential. The results showed that the aqueous and ethanol-based nanoparticles exhibited better antioxidant activity (87.94% and 88.37%) than the aqueous and ethanol crude extract (50.00% and 56.81%) at 100 μg/ ml, respectively. As the free radical scavenging effect is responsible for the blockage of ample of diseases, the plant is a potential source of nanoparticles to be a potential medicinal active agent [49,50]. As the activities of hexane extract, chloroform extract, and acetone extract-based nanoparticles are not reported, the same are estimated. The comparison of the antioxidant activities, IC50 values, and activities of the AgNPs of the aqueous extract (AQE), ethanol extract (ETE), methanol extract (MEE), n-hexane extract (NHE), chloroform extract (CLE), and acetone extract (ACE) is presented in Figure 3.
The free radical scavenging activity of the extracts of the plant and their synthesized EtOH-AgNPs was measured by the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assays. The DPPH solution of 0.1 mM concentration was prepared in ethanol for the purpose [47]. This solution (1 ml) was added to 5 ml of the crude ethanol extract at different concentrations (10, 20, 40, 80, 100, 150, and 250 μg/ ml) prepared by the dilution method. The solutions were mixed by vigorous shaking and then allowed to stand for 30 minutes at room temperature in the dark. The UV spectrum showed prominent absorption at 517 nm. Ascorbic acid was used as a standard, and the experiment was repeated three times. Antioxidant activity as percent radical scavenging activity (% RSA) by the DPPH was calculated [51]. An increase in the antioxidant activity is the measure of the decrease in the DPPH solution absorbance. The free radical scavenging effect is responsible for the blockage of ample of diseases [52,53].
The reactive oxygen species are produced during cell metabolism in both normal and pathological conditions [28]. Many diseases are caused by reactive oxygen species, such as Parkinson’s disease, cancer, cirrhosis, arthritis, asthma, and coronary heart disease [54]. Destruction of the biomolecules can be controlled by the antioxidants, as they are capable of preventing or delaying oxidative processes by inhibiting the initiation or propagation of an oxidative chain reaction [55]. They maintain the nutritional quality and increase the shelf life of food, and they are also used to prevent many oxidative-stress-related diseases. Two major synthetic antioxidants are used, such as 2, 3-ter-butyl 4-methoxyphenol and 2, 6-di-ter-butyl-4-methylphenol, but the problem with these synthetic antioxidants is that they have caused undesired health effects [56]. Thus, there is a need for the development of safe antioxidants.
Conclusion
The extracts of Rhazya stricta have shown antioxidant properties and proved to be a potential source of antioxidant agents. The extracts of the plant showed the radical scavenging properties and also proved to the antioxidant agents in a number of animal models. The results showed that the aqueous and ethanol extracts-based nanoparticles exhibited better antioxidant activity (87.94% and 88.37%) than the aqueous and ethanol crude extract (50.00% and 56.81%) at 100 μg/ ml, respectively. The plant-based NPs have also shown antioxidant activity in the DPPH assay. The plant-based AgNPs displayed a promising effect against the DPPH in a fixation-dependent way. The concentration of the extracts from 10 to 250 μg/ ml and corresponding antioxidant activities showed a linear relationship. Generally, the NPs showed better activity than the crude ethanol extract. The investigations on the extracts of the plant to determine their antioxidant potential indicate that the plant functions as a natural antioxidant source. Particularly, antioxidant activities of the nanoparticles of the extracts of different parts of the plant in different solvents need to be investigated to determine the full potential of the plant-based nanoparticles, and this may form the basis of future research.
Acknowledgement
This review is extracted from the referenced articles. The authors of this review paper duly acknowledge the work of the authors of the referred articles.
References
- Sharma R, Amin H, Galib R, Prajapati PK. Molecular targets of common Ayurvedic herbal antioxidants. J Ayurvedic Herbal Med. 2017;3(1):36–40. Available from: https://www.ayurvedjournal.com/JAHM_201731_06.pdf
- Harman D. Free radical theory of aging: dietary implications. Am J Clin Nutr. 1972;25:839–43. Available from: https://doi.org/10.1093/ajcn/25.8.839
- Harman D. Aging: phenomenon and theories. Ann N Y Acad Sci. 1998;854:1–7. Available from: https://doi.org/10.1111/j.1749-6632.1998.tb09886.x
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–47. Available from: https://ift.onlinelibrary.wiley.com/doi/abs/10.1111/j.1541-4337.2011.00156.x
- Lu LY, Ou N, Lu QB. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci Rep. 2013;3:3169. Available from: https://doi.org/10.1038/srep03169
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005;579:200–13. Available from: https://doi.org/10.1016/j.mrfmmm.2005.03.023
- Chaudière J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–62. Available from: https://doi.org/10.1016/s0278-6915(99)00090-3
- Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–8. Available from: https://doi.org/10.1111/j.1749-6632.1999.tb07814.x
- Hinneburg I, Dorman HJD, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–9. Available from: https://doi.org/10.1016/j.foodchem.2005.03.028
- Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–67. Available from: https://doi.org/10.1002/hep.24433
- Hamza AA, Ahmed MM, Elwey HM, Amin A. Melissa officinalis protects against doxorubicin-induced cardiotoxicity in rats and potentiates its anticancer activity on MCF-7 cells. PLoS One. 2016;11:e0167049. Available from: https://doi.org/10.1371/journal.pone.0167049
- Hooker JD. Flora of British India. Vol. III. London: Reeve and Company; 1975. p. 640. Available from: https://www.biodiversitylibrary.org/bibliography/678
- Wall G. A dictionary of the economic products of India. London: W. H. Allen and Company; 1982.
- Gillani SA, Kikuchi A, Shinwari ZK, Khattak ZI, Watanabe KN. Phytochemical, pharmacological and ethnobotanical studies of Rhazya stricta Decne. Phytother Res. 2007;221:301–7. Available from: https://doi.org/10.1002/ptr.2064
- Albeshri A, Baeshen NA, Bouback TA, Aljaddawi AA. A review of Rhazya stricta Decne phytochemistry, bioactivities, pharmacological activities, toxicity and folkloric medical uses. Plants (Basel). 2021;10(11):2508. Available from: https://doi.org/10.3390/plants10112508
- Bawazeer S, Rauf A, Emran TB, Aljohani ASM, Alhumaydhi FA, Khan Z, et al. Biogenic synthesis of silver nanoparticles using Rhazya stricta extract and evaluation of its biological activities. J Nanomater. 2022;2022:7365931. Available from: https://doi.org/10.1155/2022/7365931
- Iqbal S, Bhanger MI, Akhtar M, Anwar F, Ahmed KR, Anwer T. Antioxidant properties of methanolic extracts from leaves of Rhazya stricta. J Med Food. 2006;9(2):270–5. Available from: https://doi.org/10.1089/jmf.2006.9.270
- Al-Dabbagh B, Elhaty IA, Al-Hrout A, Al-Sakkaf R, Al-Awady R, Ashraf SS, et al. Antioxidant and anticancer activities of Trigonella foenumgraecum, Cassia acutifolia, and Rhazya stricta. BMC Complement Altern Med. 2018;18(1):1–12. Available from: https://doi.org/10.1186/s12906-018-2285-7
- Ali BH. The effect of treatment with the medicinal plant Rhazya stricta Decne on gentamicin nephrotoxicity in rats. Phytomedicine. 2002;9(5):385–9. Available from: https://doi.org/10.1078/09447110260571607
- Iqbal S, Bhanger MI, Akhtar M, Anwar F, Ahmed KR, Anwer T. Antioxidant properties of methanolic extracts from leaves of Rhazya stricta. J Med Food. 2006;9(2):270–5. Available from: https://doi.org/10.1089/jmf.2006.9.270
- Marwat SK, Fazal-ur-Rehman, Usman K, Shah SS, Anwar N, Ullah I. A review of phytochemistry, bioactivities and ethno medicinal uses of Rhazya stricta Decne (Apocynaceae). Afr J Microbiol Res. 2012;6(8):1629–41. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/20123132408
- Sultana N, Khalid A. Phytochemical and enzyme inhibitory studies on indigenous medicinal plant Rhazya stricta. Nat Prod Res. 2010;24:305–14. Available from: https://doi.org/10.1080/14786410802417040
- Ali BH, Bashir AK, Tanira MOM. The effect of Rhazya stricta Decne. a traditional medicinal plant, on the forced swimming test in rats. Pharmacol Biochem Behav. 1998;59:547–50. Available from: https://doi.org/10.1016/s0091-3057(97)00470-x
- Ali BH, Alqarawi AA, Bashir AK, Tanira MO. Antioxidant action of extract of the traditional medicinal plant Rhazya stricta Decne in rats. Phytother Res. 2000;14(6):469–71. Available from: https://doi.org/10.1002/1099-1573(200009)14:6%3C469::aid-ptr612%3E3.0.co;2-w
- Albarrak SM. Antioxidant and immune responses of broiler chickens supplemented with Rhazya stricta extract in drinking water. Vet World. 2021;14(6):1437–49. Available from: https://www.veterinaryworld.org/Vol.14/June-2021/6.html
- Bukhari NA, Al-Otaibi RA, Ibhrahim MM. Phytochemical and taxonomic evaluation of Rhazya stricta in Saudi Arabia. Saudi J Biol Sci. 2017;24(7):1513–21. Available from: https://doi.org/10.1016/j.sjbs.2015.10.017
- Mahmood R, Malik F, Shamas S, Ahmed T, Kuusar M, Rabnawaz S, Ashfaq M, et al. Pharmacological evaluation of Rhazya stricta root extract. Bol Latinoam Caribe Plant Med Aromat. 2020;19(2):188–206.
- Khan MJ, Baloch NU, Nabi S, Ahmed N, Bazai Z, Yasinzai M, et al. Antileishmanial, cytotoxic, antioxidant activities and phytochemical analysis of Rhazya stricta Decne leaves extracts and its fractions. Asian J Plant Sci Res. 2012;2(5):593–8. Available from: https://www.researchgate.net/publication/361366726_Antileishmanial_cytotoxic_antioxidant_activities_and_phytochemical_analysis_of_Rhazya_stricta_Decne_leaves_extracts_and_its_fractions
- Haq IU, Ullah N, Bibi G, Kanwal S, Ahmad MS, Mirza B. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran J Pharm Res. 2012;11(1):241–9. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3813110/
- Khan H, Shahzad MA, Marwat FUR, Ullah H, Mangi AA, Arain SP, et al. Phytochemical and antibacterial evaluation of Rhazya stricta Decne. Int J Biol Pharm Allied Sci. 2019;8(3):491–9. Available from: https://ijbpas.com/pdf/2019/March/MS_IJBPAS_2019_4649.pdf
- Amin A, Mousa M. Merits of anti-cancer plants from the Arabian Gulf region. Cancer Ther. 2007;5:55–66. Available from: https://www.researchgate.net/publication/233550657_Merits_of_anti-cancer_plants_from_the_Arabian_Gulf_Region
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–59. Available from: https://doi.org/10.1021/jf051513y
- Popova M, Bankova V, Butovska D, Petkov V, Nikolova-Damyanova B, Sabatini AG, Marcazzan GL, et al. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal. 2004;15(4):235–40. Available from: https://doi.org/10.1002/pca.777
- Lanjvani AH, Gangho AB, Khuhawar TMJ. Phytochemical analysis and biological activity of different parts of Rhazya stricta. Rawal Med J. 2018;43(3):532–5. Available from: https://www.rmj.org.pk/index.php?mno=288972
- Lim YY, Quah EPL. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007;103:734–40. Available from: https://doi.org/10.1016/j.foodchem.2006.09.025
- Bibi G, Haq I, Ullah N, Mannan A, Mirza B. Antitumor, cytotoxic and antioxidant potential of Aster thomsonii extracts. Afr J Pharm Pharmacol. 2011;5(2):252–8. Available from: https://www.researchgate.net/publication/260753361_Antitumor_cytotoxic_and_antioxidant_potential_of_Aster_thomsonii_extracts
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. Available from: https://doi.org/10.1016/s0891-5849(98)00315-3
- Aljawdah HM, Murshed M, Ammari AA, Maodaa SN, Al-Quraishy S. Phytochemical analysis of Rhazya stricta collected from Riyadh, Saudi Arabia as antioxidant and against Eimeria perforans activity. Indian J Anim Res. 2025:1–7. Available from: https://arccjournals.com/journal/indian-journal-of-animal-research
- Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–36. Available from: https://researchoutput.csu.edu.au/en/publications/phenolic-compounds-and-their-role-in-oxidative-processes-in-fruit
- Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. Available from: https://doi.org/10.1023/a:1007078414639
- Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9. Available from: https://www.researchgate.net/publication/222821604_Antioxidant_activity_of_phenolic_compounds_in_32_selected_herbs
- Saif A. Phytochemical, antioxidant, and antimicrobial effects of methanolic leaf extract of Rhazya stricta Decne. Int J Res Med Sci. 2022;10(2):293–300. Available from: https://dx.doi.org/10.18203/2320-6012.ijrms20220271
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–41. Available from: https://doi.org/10.1006/abio.1999.4019
- Al-Busafi S, Al-Riyami M, Al-Ouwaisi K, Hisham A. Screening of antioxidant and radical scavenging activities of some Omani medicinal plants. Sultan Qaboos Univ J Sci. 2007;12:1–6. Available from: https://squjs.squ.edu.om/cgi/viewcontent.cgi?article=1309&context=squjs
- Jafri L, Saleem S, Haq IU, Ullah N, Mirza B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arab J Chem. 2014;19:158–63. Available from: http://dx.doi.org/10.1016/j.arabjc.2014.05.002
- Basu S, Maji P, Ganguly J. Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of Nyctanthes arbortristis. Appl Nanosci. 2016;6(1):1–5. Available from: https://link.springer.com/article/10.1007/s13204-015-0407-9
- Tailer CS, Anju G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn leaves. Am J Ethnomed. 2014;1(4):244–9. Available from: https://www.researchgate.net/publication/285525623_Antioxidant_Activity_by_DPPH_Radical_Scavenging_Method_of_Ageratum_conyzoides_Linn_Leaves
- Al-Mahmud Z, Emran TB, Qais N, Bachar SC, Sarker M, Uddin MMN. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb). J Basic Clin Physiol Pharmacol. 2016;27(1):63–70. Available from: https://www.degruyterbrill.com/document/doi/10.1515/jbcpp-2015-0056/html
- Chopra H, Bibi S, Mishra AK, Tirth V, Yerramsetty SV, Murali SV, et al. Nanomaterials: A promising therapeutic approach for cardiovascular diseases. J Nanomater. 2022;Article ID 254155729. Available from: https://doi.org/10.1155/2022/4155729
- Aziz AT, Alshehri MA, Alanazi NA, Panneerselvam C, Trivedi S, Maggi F, et al. Phytochemical analysis of Rhazya stricta extract and its use in fabrication of silver nanoparticles effective against mosquito vectors and microbial pathogens. Sci Total Environ. 2020;700:134443. Available from: https://doi.org/10.1016/j.scitotenv.2019.134443
- Al-Mahmud Z, Emran TB, Qais N, Bachar SC, Sarker M, Uddin MMN. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn leaves. J Basic Clin Physiol Pharmacol. 2016;27(1):63–70. Available from: https://www.degruyterbrill.com/document/doi/10.1515/jbcpp-2015-0056/html
- Bilal M, Rasheed T, Iqbal HMN, Li C, Hu H, Zhang X. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int J Biol Macromol. 2017;105(1):393–400. Available from: https://doi.org/10.1016/j.ijbiomac.2017.07.047
- Halliwell TG. Free radicals in biology and medicine. Oxford: Oxford University Press; 2003. p. 1357–65.
- Howell JF. Food antioxidants, international perspectives: Welcome and introductory remarks. Food Chem Toxicol. 1986;24(10–11):997–1255.
- Gerber M, Boutron-Ruault MC, Hercberg S, Riboli E, Scalbert A, Siess MH. Food and cancer: State of art about the protective effect of fruits and vegetables. Bull Cancer. 2002;89(3):293–312. Available from: https://pubmed.ncbi.nlm.nih.gov/11940469/
- Namiki M. Antioxidant antimutagens in food. Crit Rev Food Sci Nutr. 1990;29(4):273–300. Available from: https://doi.org/10.1080/10408399009527528
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
