International Journal of Pharmaceutical Sciences and Developmental Research
Mycobacterium abscessus subsp. Bolletti-associated osteomyelitis of the lumbar vertebrae in immunocompetent patient
Anda Vīksna1,2, Jānis Zeltiņš1,2, Guntars Mauriņš2, Vija Riekstiņa2,3 and Iveta Ozere1,2*
2Riga East University Hospital, Latvia
3University of Latvia, Latvia
Cite this as
Vīksna A, Zeltiņš J, Mauriņš G, Riekstiņa V, Ozere I (2024) Mycobacterium abscessus subsp. Bolletti-associated osteomyelitis of the lumbar vertebrae in immunocompetent patient. Int J Pharm Sci Dev Res 10(1): 006-009. DOI: 10.17352/ijpsdr.000051Copyright License
© 2024 Vīksna A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Nontuberculous mycobacteria (NTM)-related Vertebral Osteomyelitis (VO) is a rare disease with challenging diagnosis and management. NTM-caused disease more often develops in vulnerable populations, affecting individuals who are immunocompromised or who have underlying health conditions.
Methods: We report a case of Mycobacterium abscessus subsp. Bolletti-associated VO in a 60-year-old immunocompetent female patient.
Results: The patient presented with chronic back pain and paresis of the legs. She underwent surgery - partial resection of L4 and L5 vertebral bodies, and vertebroplasty with allograft. Culture for Mycobacterium abscessus subspace Bolletti was positive from operation material, and treatment with multiagent antibacterial therapy was started. After 2 months of treatment, an asymptomatic m.psoas abscess was identified on control imaging. Repeated surgery with abscess drainage was applied, and treatment of M.abscessus infection was continued. After a 12-month course of treatment, the patient recovered successfully and could walk without support. During treatment, she experienced a lot of drug side effects including peripheral neuropathy.
Conclusion: Sampling for Mycobacterium tuberculosis and NTM should be always considered in both, immunocompromised and immunocompetent patients presenting with clinical symptoms and imaging findings consistent with VO.
Abbreviations
VO: Vertebral Osteomyelitis; NTM: Nontuberculous Mycobacteria; CT: Computed Tomography; PCR: Polymerase Chain Reaction; MRI: Magnetic Resonance Imaging
Introduction
Non-tuberculous mycobacteria (NTM) are environmental opportunistic pathogens distributed widely in soil and water. More than 200 species of NTM are prevalent globally, but only a few of them have pathogenic potential. The most common NTM species causing human disease are the slow-growing mycobacteria Mycobacterium avium in the Mycobacterium avium complex, the rapid-growing M. abscessus, and M. Kansasii [1,2]. NTM-caused disease more often develops in vulnerable populations, affecting individuals who are immunocompromised or who have underlying health conditions such as congenital or acquired structural airway diseases, malnutrition, older age, and Mendelian susceptibility to mycobacterial disease. However, there is also a noted increased incidence of NTM disease in people who are not immunocompromised and without any preexisting lung diseases [3,4]. The most common clinical syndrome is pulmonary infection, which accounts for about 90% of NTM-related diseases. However, osteoarticular, disseminated, skin, and soft tissue infections have increasingly been reported in the last decade [5,6]. Diagnosing of extrapulmonary NTM is challenging, and management is complex comprising long courses of combination antibiotic regimens and may require adjuvant surgical interventions [6].
Here we report a case of vertebral osteomyelitis caused by Mycobacterium abscessus subsp. Bolletti in immunocompetent patient.
Case presentation
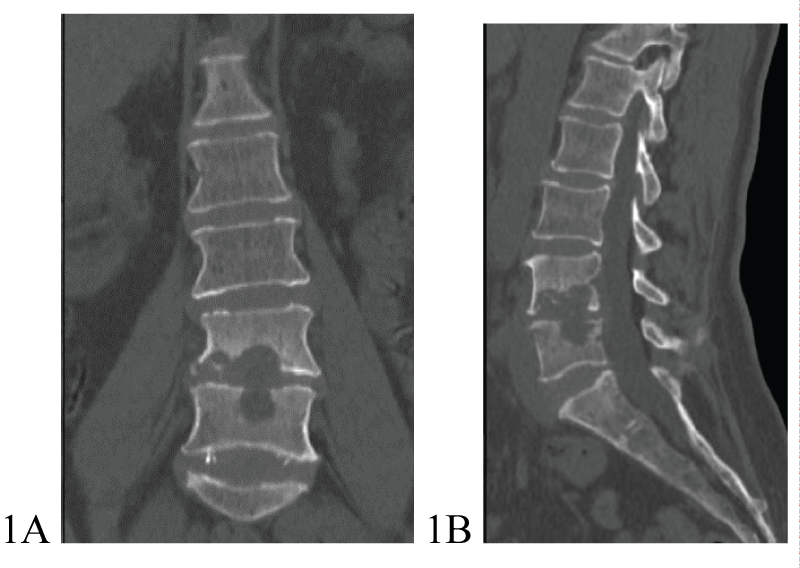
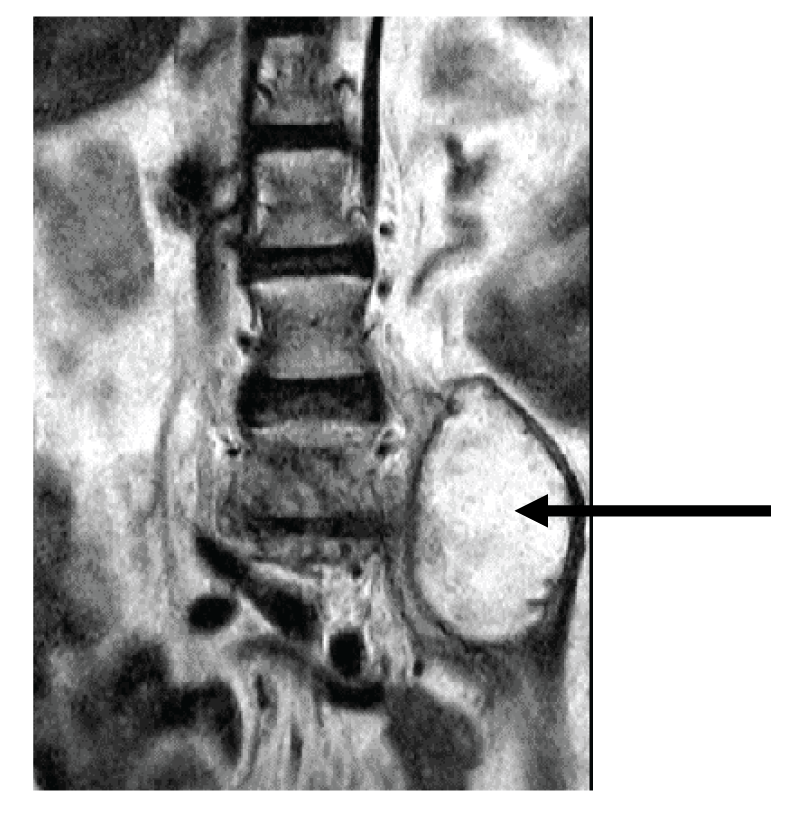
A 60-year-old female patient was hospitalized at Riga East University Hospital on March 23, 2023, due to severe pain in the lumbar spine that started 3 months before hospitalization, and paresis in the legs that appeared on the day of hospitalization. Spinal Computed Tomography (CT) revealed extensive destruction in lumbar L4-L5 vertebrae and intervertebral disc (Figures 1A,1B). A chest CT showed no signs indicative of pulmonary infection. On March 29, 2023, the patient underwent surgery - left-sided lumbotomy, partial resection of L4 and L5 vertebral bodies, and vertebroplasty with allograft. Histopathological investigation showed osteonecrosis and acute non-specific spondylitis in L4 and L5 vertebral bodies. Initial smear testing for acid-fast bacilli and real-time PCR by GeneXpert ®MTB/RIF method from operational material was negative. On April 6, 2023, grows of nontuberculous mycobacteria from operational material was detected, and species identification confirmed the presence of Mycobacterium abscessus subspace Bolletti. Mutations in genes rrs and rrl were not identified, however, mutation in gene erm (41) T-28 responsible for resistance to macrolides was positive. Treatment of M.abscessus infection with imipenem/cilastatin, moxifloxacin, clofazimine, and amikacin 3 times per week was started on April 21, 2023 (Table 1). Within two months after starting treatment, the patient's clinical condition improved significantly – she did not experience significant pain in the lumbar spine, was neurologically intact, and was able to walk with the help of a walker. Despite clinical improvement control MRI on June 29, 2023, showed left-sided m.psoas abscess formation (Figure 2). The patient received repeated surgery on July 4, 2023. During surgery, a connection between the abscess and L4-L5 intervertebral disc position was identified. Abscess drainage, resection of residual pathological tissue of the L4-L5 intervertebral disc, and vertebroplasty with allograft were performed. Histopathology of the second operational material confirmed destructive spondylodiscitis typical for mycobacterial infection. The culture for M.abscessus from the second operational material was negative. The patient remained neurologically intact after the second surgery and her functionality continued to improve.
The patient experienced a lot of side effects from long-term multi-agent therapy.
Amikacin was stopped due to the development of sensoneural hearing impairment at high frequencies on an audiogram. Linezolid was stopped because of peripheral neuropathy and anaemia (in total patient received linezolid for 5 months).
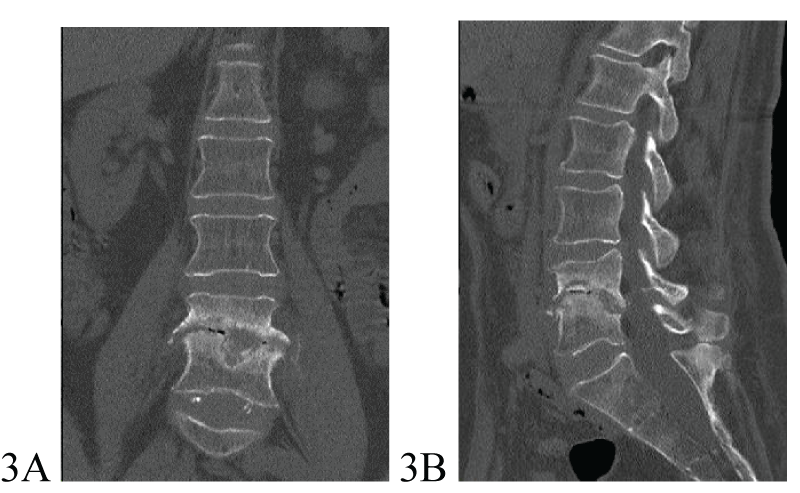
Moxifloxacin was discontinued because of the prolongation of QTc interval on EKG. The development of hepatoxicity was a reason for the temporary discontinuation of all drugs from December 15 up to January 4. A follow-up radiological examination was performed on March 26, 2024 (after 11 months of treatment). Spinal canal stenosis at L3-L4 and L4-L5 levels and destructive changes of the L4-L5 vertebrae with sclerotic vertebral body edges were revealed and interpreted as residual changes after vertebral destruction and surgery (Figures 3A,3B).
Despite residual structural alterations of the lumbar vertebrae, the patient remained neurologically intact and had excellent clinical improvement with preserved functionality – she walked without support and did not use pain relief medications regularly.
However, the patient continued to suffer from peripheral neuropathy, despite linezolid being stopped at 7th month of treatment.
Discussion
Timely recognition of VO, identification of its exact etiology, and use of adequate antimicrobial therapy are cornerstones for a patient’s survival and quality of life. The VO diagnosis with NTM is dependent on the existing examination modalities, such as clinical presentation, imaging, histopathological findings, and microbiological examination results. Optimally, vertebral osteomyelitis should be diagnosed before destruction has developed in the vertebral bodies and intervertebral discs. Although at this early stage of the disease, CT or MRI is not always performed, and if performed, early radiological signs of vertebral osteomyelitis are not consistently recognized.
Clinical manifestation of VO is non-specific. The most common symptom is back pain which is consistent with the level of the involved vertebra [7]. Although back pain is a common problem that can have many different causes, it is very challenging for a doctor to think about and diagnose the patient with VO at this stage, while the patient is neurologically intact.
Imaging findings are fundamental for the diagnosis of VO. NTM vertebral infection could result in bone destruction of the articular surface, accompanied by marginal sclerosis, and may be associated with abscess formation. Contrast-enhanced MRI is the gold standard for diagnosing VO due to its high sensitivity (96%), high specificity (94%), and ability to provide detailed data on paraspinal soft tissues and the epidural space CT is useful for detecting bony sequestra and soft tissue abscesses [8-10].
Imaging findings of VO caused by nontuberculous mycobacteria were reviewed by Yu et al. Authors pointed out, that early diagnosis of NTM vertebral infection should be considered when multiple lesions with osteosclerosis and articular surface destruction with sclerotic edges are observed alongside persistent inflammatory symptoms [10].
Definitive diagnosis of VO depends on tissue sampling for culture and histopathologic investigation. VO is most often a single pathogen infection, and Staphylococcus aureus is the most common causative agent. In immunocompromised patients, Mycobacterium avium complex mycobacteria is a common causative pathogen, in endemic region Mycobacterium tuberculosis and Brucella, and very rarely endemic fungal infections (blastomycosis and histoplasmosis) can cause VO [9,11].
VO caused by NTM, and particularly Mycobacterium abscessus in immunocompetent patients is extremely rare. It is generally acknowledged that people with immunodeficiency or long-term hormone use are susceptible to NTM infections [7,10].
Treatment of extrapulmonary NTM infections consists of a combination of antimicrobial therapy and potential source control surgery for an optimal outcome. Antimicrobial therapy varies depending on the species, the extent of disease, underlying comorbidities, and drug susceptibility testing. However, there is a lack of consensus on antimicrobial agents, their combination, and the duration of treatment for VO caused by M.abscessus infection [6,7,12].
While there are currently no established guidelines specifically for extrapulmonary NTM disease, clinicians can refer to the joint ATS/ERS/ESCMID/IDSA guidelines recommendations for less common NTM isolates, both focusing on NTM pulmonary disease, to guide their antimicrobial regimen [7].
M. abscessus isolates display in vitro resistance to most oral antibiotics and is generally susceptible to a limited number of parenteral agents including tigecycline, imipenem, cefoxitin, and amikacin. In M. abscessus pulmonary disease the association between in vitro drug susceptibility and in vivo treatment outcome is evident for macrolides and amikacin. Parenteral drugs with in vitro activity include amikacin, imipenem, cefoxitin, and tigecycline. Oral drugs with some activity are macrolides, linezolid, and clofazimine. Clofazimine shows in vitro activity, acts synergistically with amikacin and macrolides, and prevents the emergence of amikacin-resistant M. abscessus in vitro. Strains of M. abscessus subsp. Bolletii has an erythromycin resistance methylase (erm) gene, named erm (41), that results in inducible resistance to macrolides. All of the 3 M. abscessus subspecies can develop constitutive macrolide resistance owing to 23S RNA (rrl) gene mutations. When macrolide resistance is documented, ≥ 4 drugs should be administered during the initial intensive phase of treatment. Azithromycin and clarithromycin are not counted as active drugs in the setting of resistance or inducible resistance but may be used for their immunomodulatory effects [12].
In our patient the diagnosis of VO was late regarding clinical symptoms and imaging findings: patient presented with neurological complications and extensive destruction of L4-L5 vertebral bodies and intervertebral disc at diagnosis.
Immunocompromising conditions and underlying diseases were not identified in our patient. The only comorbidities were chronic kidney disease and primary arterial hypertension, so there was a low index of suspicion for opportunistic pathogens. Retrospectively infection with M.abscessus could be explained by her regular contact with the soil and plants due to her occupation, gardening. However, skin wounds or traumatic lesions were not recognized by the patient.
VO was identified on CT scanning, and its etiology was confirmed by positive culture for M.abscessus subsp. Bolletti. Mutation in the erm gene responsible for resistance to macrolides was identified. Phenotypic drug susceptibility testing of nontuberculous mycobacteria was not available. The initial intensive phase of treatment included 4 drugs with assumed activity against M.abscessus, followed by the continuation phase of treatment with 3 drugs. Clarithtromycin was included due to its immunomodulatory potential. Unfortunately, our patient experienced all available drug side effects necessitating regimen corrections and even short interruptions. Despite challenging treatment, the patient recovered, and her treatment outcome was satisfactory.
Conclusion
NTM-related VO is a rare disease that more often affects immunocompromised patients. However, there is also a noted increased incidence of NTM disease in people who are not immunocompromised and without any underlying health conditions. The outcome of the disease depends on timely precise diagnosis and appropriate treatment. Definitive diagnosis of VO is based on tissue sampling for culture and histopathologic investigation. Despite Staphylococcus aureus still being the most common causative agent, sampling for Mycobacterium tuberculosis and NTM should be always considered in both, immunocompromised and immunocompetent patients presenting with clinical symptoms and imaging findings consistent with VO.
Ethical considerations
The patient has given verbal consent for the case description and publication.
Conceptualization, A.V., I.O..; original draft preparation, A.V., I.O.; review and editing, J.Z., G.M., V.R. All authors reviewed the manuscript. The authors have not received grant support.
- Kalpana T, Mugunthan M, Joseph NM, Ellappan K. A Comprehensive Review and Update on Epidemiology, Symptomatology and Management of Nontuberculous Mycobacteria (NTM). J Pure Appl Microbiol. 2022;16(2):814-824. doi: 10.22207/JPAM.16.2.41
- Kim JH, Jung IY, Song JE, Kim EJ, Kim JH, Lee WJ, Seong H, Ahn JY, Jeong SJ, Ku NS, Choi JY, Yeom JS, Song YG. Profiles of Extrapulmonary Nontuberculous Mycobacteria Infections and Predictors for Species: A Multicenter Retrospective Study. Pathogens. 2020 Nov 14;9(11):949. doi: 10.3390/pathogens9110949. PMID: 33202553; PMCID: PMC7697751.
- To K, Cao R, Yegiazaryan A, Owens J, Venketaraman V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J Clin Med. 2020 Aug 6;9(8):2541. doi: 10.3390/jcm9082541. PMID: 32781595; PMCID: PMC7463534.
- Errami A, El Baghdadi J, Ailal F, Benhsaien I, Ouazahrou K, et al. Mendelian susceptibility to mycobacterial disease: an overview. Egypt J Med Hum Genet. 2023; 24:7. https://doi.org/10.1186/s43042-022-00358-x.
- Dahl VN, Mølhave M, Fløe A, van Ingen J, Schön T, Lillebaek T, Andersen AB, Wejse C. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis. 2022 Dec;125:120-131. doi: 10.1016/j.ijid.2022.10.013. Epub 2022 Oct 13. PMID: 36244600.
- Kiselinova M, Naesens L, Huis In 't Veld D, Boelens J, Van Braeckel E, Vande Weygaerde Y, Callens S. Management Challenges of Extrapulmonary Nontuberculous Mycobacterial Infection: A Single-Center Case Series and Literature Review. Pathogens. 2023 Dec 21;13(1):12. doi: 10.3390/pathogens13010012. PMID: 38276158; PMCID: PMC10819148.
- Moral MZ, Desai K, Arain AR, O'Leary RE, Haddad SF, Lawrence JP. Mycobacteriumabscessus-associated vertebral osteomyelitis in an immunocompetent patient: a rare case report and literature review. Spinal Cord Ser Cases. 2019 May 31;5:53. doi: 10.1038/s41394-019-0197-5. PMID: 31632711; PMCID: PMC6786306.
- Elbahr SU, Ozdemir YE, Karaali R, Balkan II, Saltoglu N, et al. Vertebral Osteomyelitis: What has Changed in Last 10 Years? Phnx Med J. 2023;5(2):87-93.
- Mehkri Y, Felisma P, Panther E, Lucke-Wold B. Osteomyelitis of the spine: treatments and future directions. Infect Dis Res. 2022;3(1):3. doi: 10.53388/IDR20220117003.
- Yu XJ, Lin YD, Hu P, Zee CS, Ji SJ, Zhou F. Imaging findings of vertebral osteomyelitis caused by nontuberculous mycobacterial organisms: Three case reports and literature review. Medicine (Baltimore). 2022 Jun 17;101(24):e29395. doi: 10.1097/MD.0000000000029395. PMID: 35713445; PMCID: PMC9276087.
- Graeber A, Cecava ND. Vertebral Osteomyelitis. [Updated 2023 Jul 17]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024 Jan. https://www.ncbi.nlm.nih.gov/books/NBK532256/
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020 Jul 7;56(1):2000535. doi: 10.1183/13993003.00535-2020. PMID: 32636299; PMCID: PMC8375621.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
