Open Journal of Pharmacology and Pharmacotherapeutics
Effect of Prolonged-acting Insulin on Intestinal and Hepatic Metabolism of 4-nitrophenol in STZ-Induced Experimental Diabetes
1Regulatory Affairs Department, Gedeon Richter România S.A., Târgu Mureș, Romania
2Institute of Pharmaceutical Chemistry, Pharmaceutical Faculty, University of Pécs, Hungary
3Department of Pharmacology and Pharmacotherapy, Medical Faculty, University of Pécs, Hungary
Author and article information
Cite this as
Kovacs NP, Perjési P, Fischer E, Almási A. Effect of Prolonged-acting Insulin on Intestinal and Hepatic Metabolism of 4-nitrophenol in STZ-Induced Experimental Diabetes. Open J Pharmacol Pharmacother. 2025; 10(1): 014-019. Available from: 10.17352/ojpp.000027
Copyright License
© 2025 Kovacs NP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Metabolism of xenobiotics can be influenced by a number of factors, among which hyperglycaemia caused by the insufficiency of the glucose metabolism (e.g., diabetes mellitus, impaired glucose tolerance, resistance) should be emphasized. Not only the pathological condition, but the compounds administered as treatment can alter the metabolic profile of certain pharmacons. While the rapid-acting insulin exerts its effect mainly with the acute change of the blood glucose level, the prolonged-acting insulin affects in a more complex way.
Because of its simple and well-characterised metabolic profile, the p-nitrophenol (PNP) is a model compound for the investigation of conjugative (glucuronidation and sulfation) transformations.
An in vivo experimental study was evaluated to determine the quantities of the forming p – p-nitrophenyl-glucuronide (PNP-G) and p-nitrophenol-sulfate (PNP-S) conjugates in small intestinal perfusate and bile samples in physiologic condition, in experimental diabetes (streptozotocine (STZ) induced), and in compensated diabetes with prolonged–acting insulin. In these investigations, the formed metabolic profile was influenced by the individual effect of the administered insulin.
PNP: p-nitrophenol; PNP-G: p-nitrophenyl glucuronide; PNP-S: p-nitrophenyl sulfate; UGT: UDP Glucuronosyltransferase; HPLC: High-Performance Liquid Chromatography; RP-HPLC: Reverse-Phase High-Performance Liquid Chromatography; STZ: Streptozotocin; MRP: Multidrug Resistance Protein
Introduction
At the oral drug administration, after the intestinal absorption, the molecules first reach the portal blood circulation and can be metabolized in the liver. A very important aspect of intestinal metabolism is its location, because this is the first line at the site of entry, where exogenous compounds transform and can be excreted by the enterocytes back into the intestinal lumen [1,2]. Nevertheless, the organ with the most significant metabolic capacity is the liver.
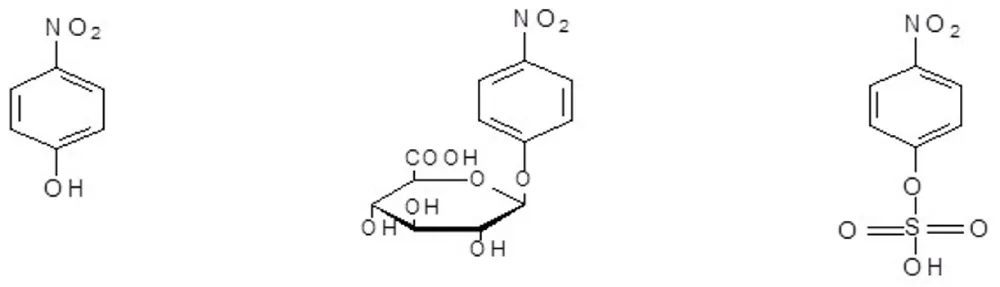
P-nitrophenol (PNP) is excreted almost exclusively as its glucuronide (PNP-G) and sulfate (PNP-S) (Figure 1) conjugates in both mammals and birds [3,4]. Because of this simple and well-characterized metabolic profile, PNP is widely used as a model substrate to evaluate the influence of drug therapy, disease, nutrient deficiencies, and other physiologically altered conditions on conjugative drug metabolism (glucururonidation and sometimes sulfation) in animal studies both in vitro and in vivo [5-7].
It needs to be mentioned that the conjugative drug metabolism occurs frequently at drug molecules containing a phenolic structural element, such as non–steroidal anti-inflammatory drugs, steroids, thyroid hormones, antidepressants [8-11]
In earlier investigations, it was concluded that diabetes mellitus, decreased glucose intolerance, resistance to insulin, and other hyperglycaemias caused by insufficiencies of the metabolism of glucose may alter the conjugative drug metabolism [12,13].
There is little data about the changes in the metabolic profile of the small intestine under the condition of diabetes and during the replacement of insulin. It is known, however, that diabetes and insulin treatment can change the protein synthesis and elimination of some drugs.
Diabetes can cause profound changes in the intrinsic synthetic processes, similar to those of insulin, applied in the replacement therapy.
Materials and methods
Chemicals
p-nitrophenol (PNP), p–nitrophenol–β–glucuronide (PNP–G), p-nitrophenol-sulfate (PNP–S), p-ethylphenol(ETP), streptozotocin (STZ), tetrabutyl-ammonium bromide( TBAB), uridine 5’-diphosphoglucuronic acid (UDPGA), prolonged-acting isophane (NPH) insulin (Humulin N), Brij56®, HEPES, and DL-dithiothreitol were obtained from the Sigma Aldrich Company (Budapest, Hungary). All other chemicals and reagents were analytical or HPLC grade. The standard isotonic perfusion medium had the following composition (mmol/l): NaCl 96.4, KCl 7.0, CaCl2 3.0, MgSO4 1.0, sodium phosphate buffer (pH 7.4) 0.9, TRIS buffer (pH 7.4) 29.5, glucose 14.0, mannitol 14.0.
Animals and experimental procedure
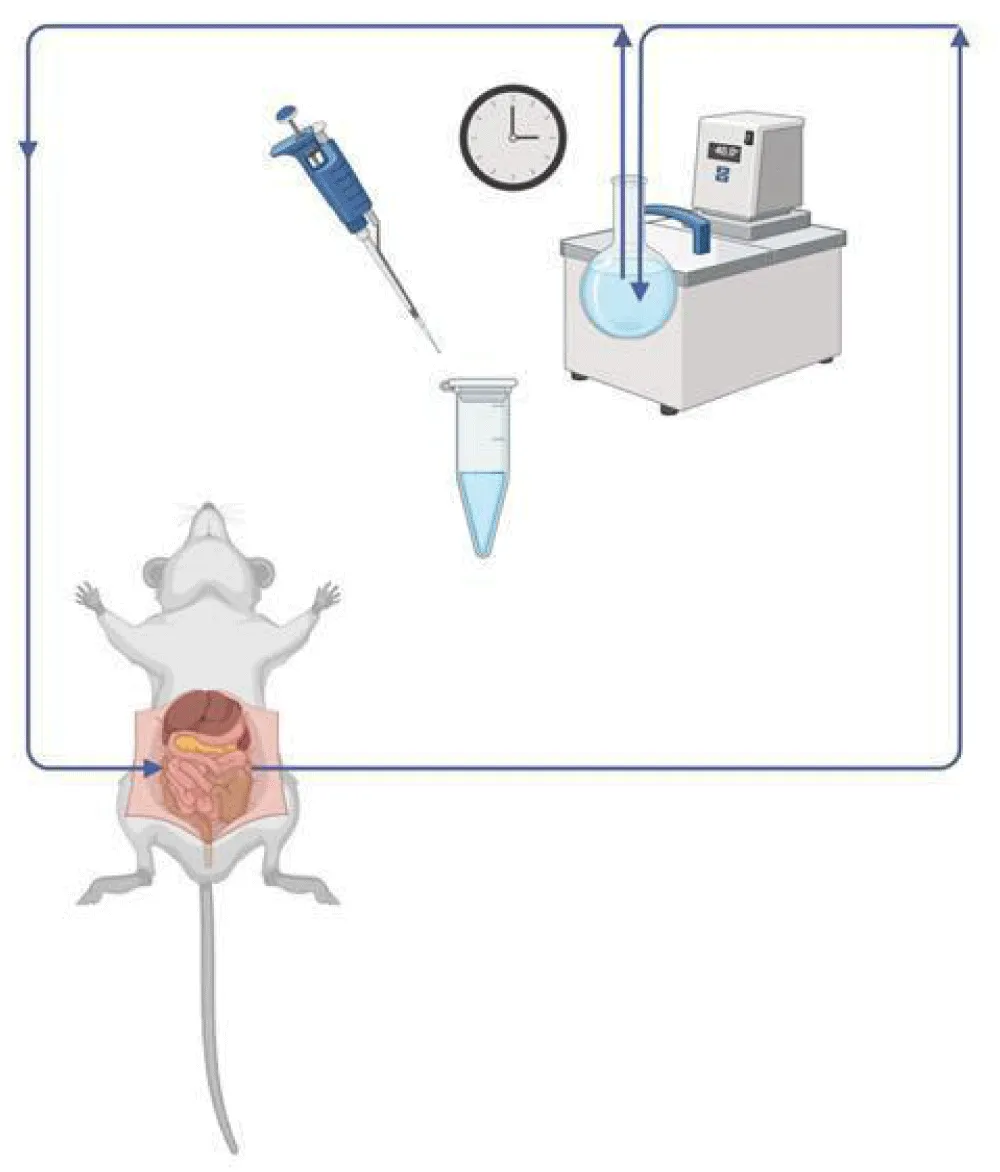
Male Wistar rats (weighing 220-250 g) were used. The animals were anesthetized with urethane (1.2 g/kg i.p.). The abdomen was opened by a midline incision, and a proximal jejunal loop (length about 10 cm) was isolated and cannulated in vivo. The lumen of the jejunal loop was gently flushed with warmed isotonic solution to remove digesta and food residues and then blown empty with 4-5 ml of air. Perfusion through the lumen of the jejunal loop with isotonic medium containing 500 µM PNP was carried out at a rate of 13 ml/min in a recirculation mode for 90 minutes. The volume of samples obtained from the perfusion medium coming out from the cannulated segments of the small intestine was 250 µl, and the initial perfusion volume was 15 ml. The temperature of the perfusion medium was maintained constant at 37 °C. (Figure 2). When the biliary excretion was investigated, the bile duct was cannulated with PE-10 tubing, and the bile was collected in 15-minute periods. The samples were stored in a refrigerator (- 20 °C) until analysis.
Experimental diabetes was induced by i.v. Administration of STZ at a dose of 65 mg/kg. The experimental procedure was performed after one week of STZ-treatment. Prolonged-acting isophane (NPH) insulin was administered in a dose of 2x15 U/kg subcutaneously (in the early morning and the early evening, respectively). Small intestinal perfusate, proximal jejunum, liver, and bile samples were obtained from the same animals of three different groups of rats (control, STZ-pretreated with or without insulin). Blood glucose measurements were performed two hours after the morning insulin administration.
Analytical conditions, instrumentation
The intestinal perfusate and bile samples were analyzed by a UV-Vis RP-HPLC method, as it was developed and published earlier (1, 3). Briefly, 50 µl of bile samples was mixed and vortexed with cold methanol in a total volume of 250 µl containing 2.5 mM ETP as an internal standard. A mixture of 0.01 M citrate buffer (pH 6.2) and methanol (47:53 v/v%) containing 0.03 M tetrabutylammonium bromide was used as a mobile phase. The flow rate of the eluent was set to 1.0 ml. min-1.
The HPLC system consisted of a Varian 2010 pump, a Rheodyne 7724i injection valve, a UV-Detector 308, for data collection and integration, a PowerChrom 280 data module, and software.
A Nucleosyl 100 C18 reversed-phase column (250 mm x 4.6 mm I.D., 10 µm particle size) and a TR-C-160K1 ODS guard column were employed for the separation and measurement of metabolites.
UV measurements were performed on a Pye Unicam (Philips) PU 8800 UV-Vis spectrophotometer (Philips, Cambridge, UK) at ambient temperature. A Mettler Toledo MP 220 pH meter and a Mettler Toledo Inlab 413 electrode (Mettler Toledo, Budapest, Hungary) were used to adjust the pH of the electrolyte solutions.
Enzyme assay
Enzyme assays were performed as it was published earlier [14] and as it is summarized in the following.
UDP-glucuronytransferase assay
Organs (jejunal intestine, liver) were obtained from rats after perfusion, perfusion after STZ pretreatment, and perfusion after STZ pretreatment with prolonged-acting insulin supplementation. All the perfusions were made with 500 µM PNP. Rats were gently dissected, and the removed organs were immediately frozen and stored at – 80 °C. 250 – 500 mg of small intestine and liver chunks were cut into pieces and placed into 2.5 - 5 ml of cold (4 °C) homogenization buffer (250 mM sucrose, 1 mM DL-dithiothreitol, 10 mM HepesTris buffer pH 8). Organs were disrupted using an Ultra-Turrax homogenizer, and the homogenization was completed by a glass dunce homogenizer.
After disruption, the organ homogenate was diluted with the homogenization buffer to have a protein concentration of 1 mg/ml. Quantification of protein was performed by the biuret assay [15].
UGT enzyme activity assays were carried out in an incubation mixture of 100 μl organ homogenate (1 mg/ml protein) and 100 μl of incubation buffer (200 mM Tris-HCl, 10 mM MgCl2, 0.1 % Brij56®, pH 7.4) containing 2 mM PNP. Half of the incubates contained 5 mM UDPGA (uridine 5’-diphosphoglucuronic acid) in the total volume. After 30 min incubation at 37 °C, the reaction was quenched by adding 3.8 ml 0.1 M ice-cold NaOH, and the absorbance of the unconjugated PNP was measured at 405 nm. Specific UGT activity was calculated based on metabolized PNP and expressed in μmol PNP/mg/min.
Calculations, statistical analysis
Enzyme activities are expressed in the amounts of substrates or metabolites (in µmol at glucuronide and in nmol at sulfate) calculated for 1 mg protein and 1 minute (details in “Enzyme assays” and “Results”).
The biliary excretion rate of PNP metabolites was calculated based on their concentration in the bile and the volume of the biliary flow. Data show the mean ± S.E. of five experiments.
Data were analysed by one-way ANOVA, and the significant differences were calculated by Student’s t-test.
All animal procedures were performed according to the Hungarian Animal Protection Act Scientific Procedures (Government Regulation 243/1998), and the study was approved by the Institutional Animal Care and Use Committee of the University of Pécs.
Results and discussion
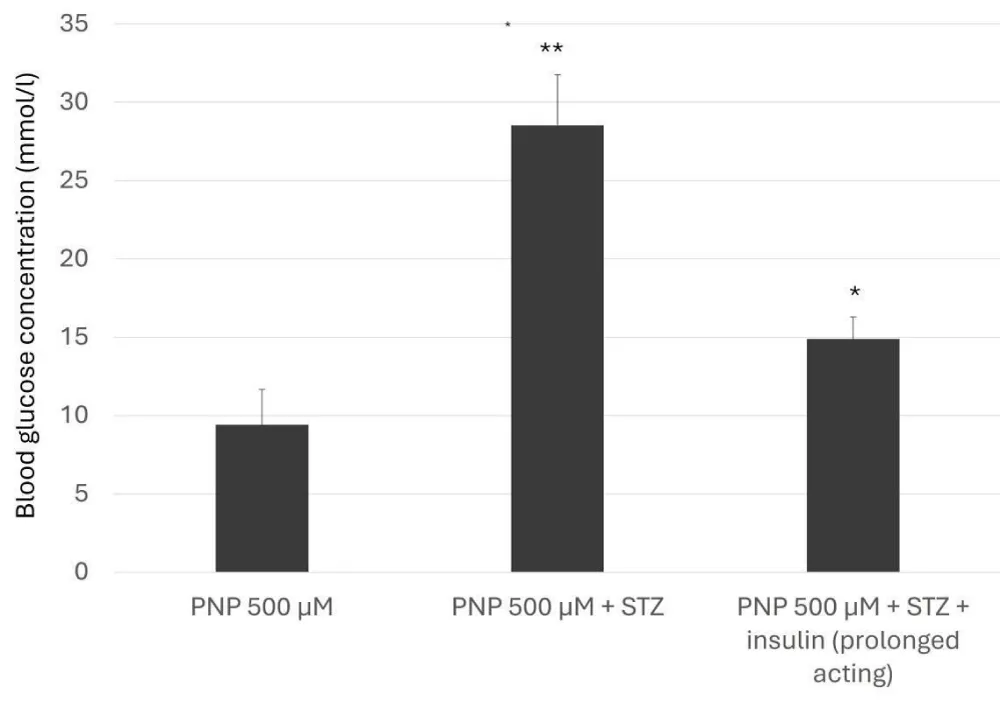
The determination of the metabolites from the small intestinal perfusate and bile was performed using the isosocratic, validated RP HPLC UV-Vis methods, which were developed for the quantification of paracetamol and its major metabolites (PNP-G and PNP-S) [16,17]. The intravenous streptozotocin pretreatment raised the blood glucose level to the diabetic range, and the applied prolonged-acting insulin held back this increase. This intervention has a slightly weaker effect on the blood glucose level than that of the acute intravenous administration of rapid-acting insulin in previous investigations [18].
With the prolonged-acting insulin, the daily one administration cannot compensate by a satisfactory degree the rising blood glucose levels. Among various combinations, the daily two (in 15 IU/kg) administrations became the most acceptable [19]. This inhibition has reduced the glucose levels, as shown in Figure 3.
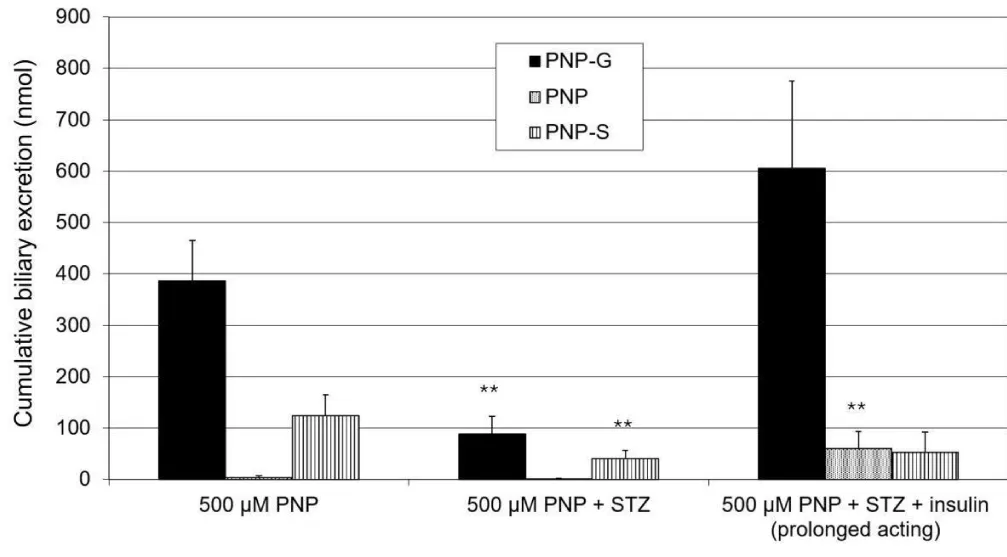
The p-nitrophenol disappears from the small intestine during the 90-minute-long experiment, and practically only the PNP-G is excreted back into the lumen. The STZ-induced diabetes can increase the quantity of the PNP-G, while the PNP-S remains constantly on the edge or below the detectability.
Earlier investigations reveal that experimental diabetes can significantly influence the enzyme activities (primarily through disruptions in insulin signaling and metabolic homeostasis), differently in various tissues. The uridin glucuronyltransferase (UGT) enzyme shows a considerable expression in the small intestinal mucosa [20,21].
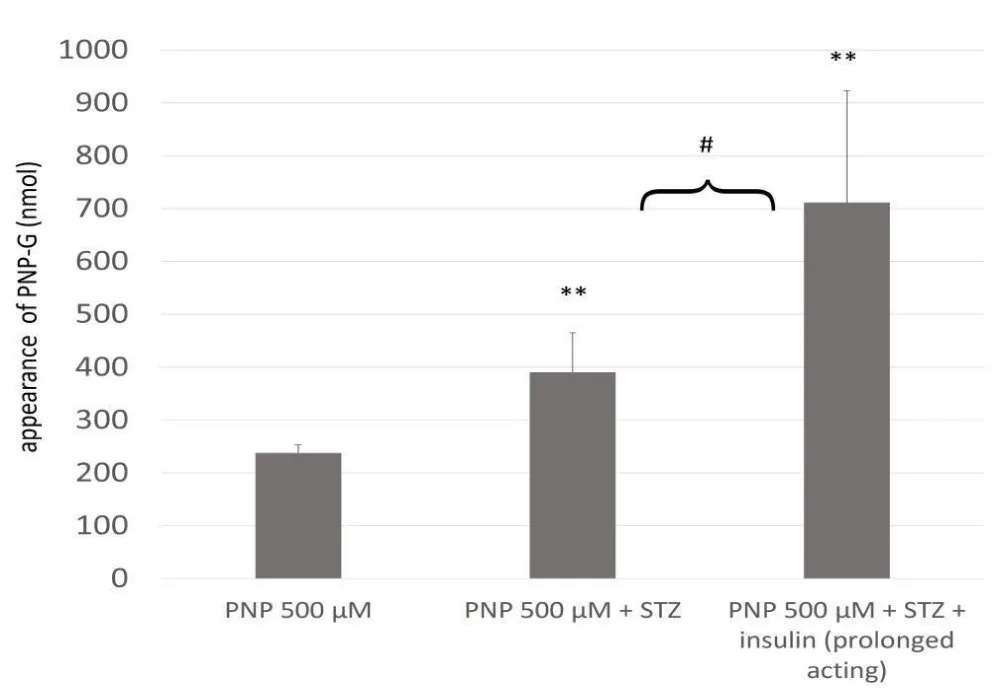
Experimental diabetes increases the formation of the excreted glucuronide in the small intestine, and the prolonged-acting insulin continues this tendency (Figure 4). Earlier experiments with rapid-acting insulin show a reduction in the level of the excreted PNP-G [18], while the UGT activities exhibit similar patterns of alteration. Meanwhile, prolonged insulin exposure can intensify the activity and the expression of the MRP2 efflux transporters [22,23].
In the bile, the PNP-G remains the dominant metabolite, the level of this excreted metabolite decreases due to the effect of the experimental diabetes, and these changes can be reversed by the effect of the prolonged-acting insulin. The PNP-S is also detectable; its presence is also associated with diabetes, and this change cannot be turned back, or only partly (Figure 5). These changes correspond with observations at the rapid-acting insulin [24]. Surprisingly, in the cases of the insulin treatment, a measurable part of unmetabolised PNP is excreted into the bile.
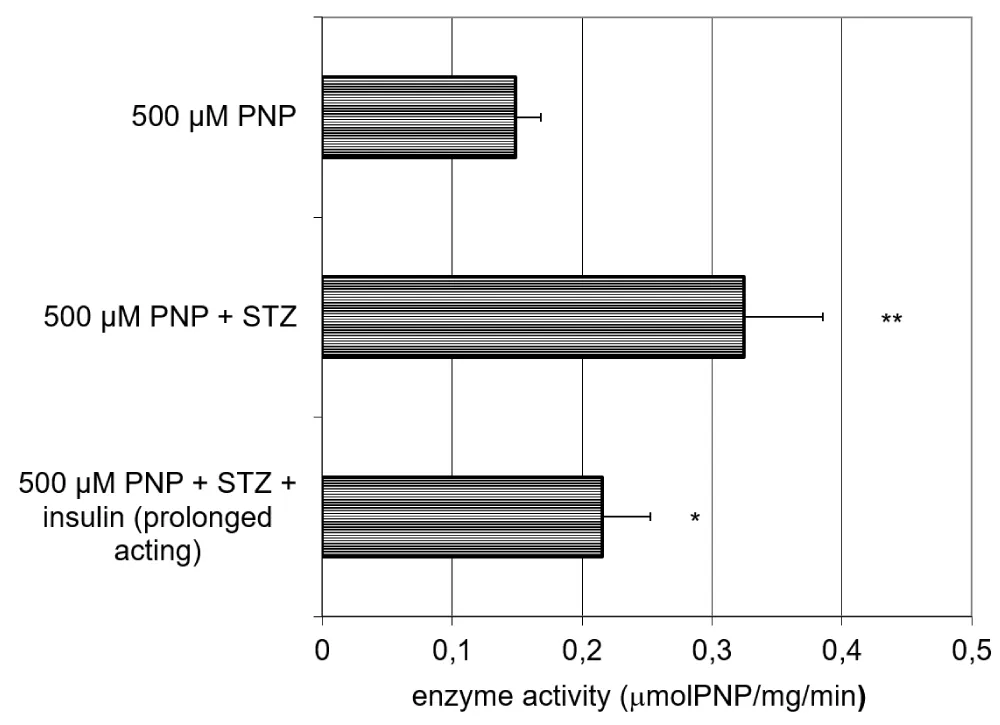
The activity of the UGT in the small intestinal homogenate increases due to the effect of the STZ pretreatment, and this increase can only partially be reversed by the effect of the prolonged-acting insulin (Figure 6).
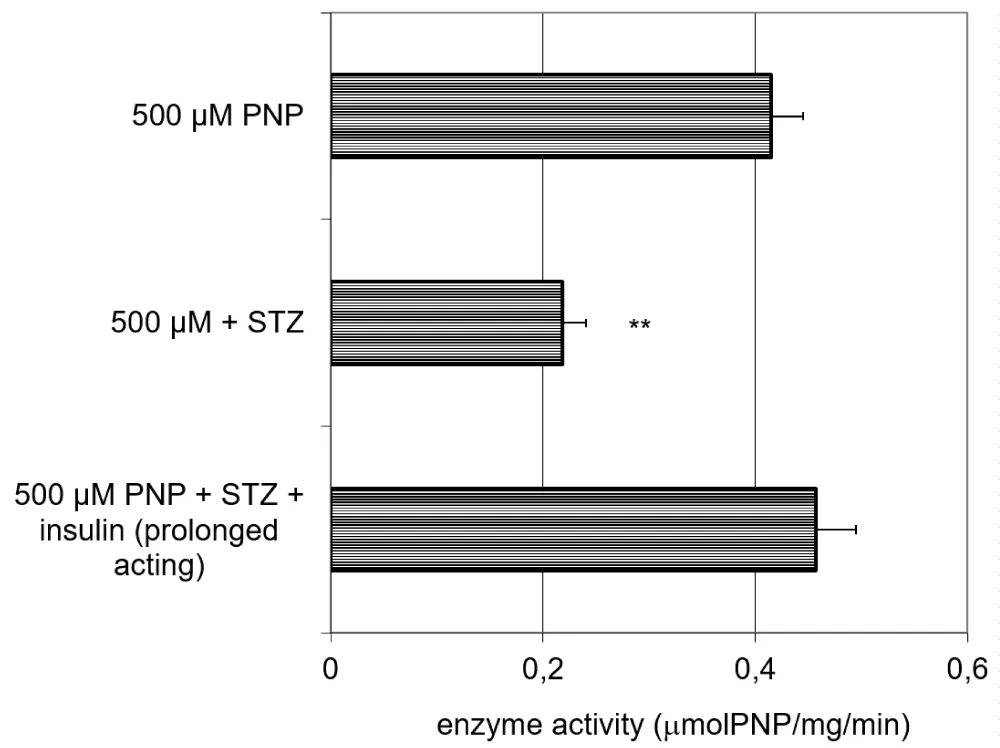
The activity of the UGT in the liver homogenate decreases due to the effect of the STZ pretreatment, and this increase can be fully reversed by the effect of the prolonged-acting insulin (Figure 7).
The change of the activity of the UDP-glucuronyltransferase in the small jejunal perfusate, partly, while at the liver largely related to the excreted amount of PNP-G.
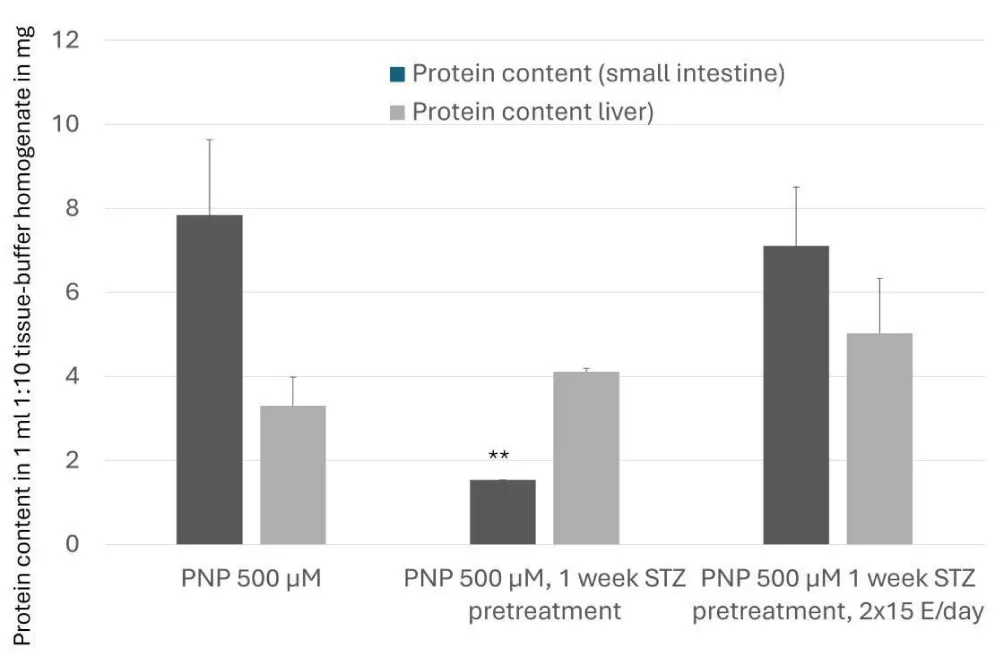
Experimental diabetes can affect the protein content in the small intestine, too. After STZ pretreatment, the protein degradation increases; meanwhile, the administration of the prolonged-acting insulin can compensate for this change. In the liver, the protein content in none of the investigated groups showed a significant alteration. In diabetes, the protein metabolism is frequently disrupted [25]. The prolonged action of insulin is associated with improvements in protein synthesis, particularly in individuals with metabolic disorders, such as diabetes (Figure 8). Moreover, the stimulation of protein synthesis by insulin is well-documented, with evidence indicating the involvement of protein kinase C (PKC) as a crucial mediator in this effect. Prolonged exposure to insulin has been linked to sustained activation of the mechanistic target of rapamycin (mTOR) pathway, which is pivotal in cellular protein synthesis and growth [26,27]. The administration of the prolonged action (isophan) insulin reverts the protein synthesis extent close to that of the control value; meanwhile, in the same arrangement, the UGT activity remains elevated (this elevation can be observed in the experiments with rapid-acting insulin, too [18].
Conclusion
The recent findings allow us to conclude that at the administration of a 500 μM dose of the PNP, the quantities of the excreted PNP-G metabolite mainly correlate with the enzyme activities in the small intestine and the liver, either. This process can be modified by the prolonged-acting insulin with its effect on the protein (enzyme) synthesis and the elevated activity and expression of transporters (MRP2), especially in the small intestine.
- Kaminsky LS, Zhang QY. The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos. 2003;31(12):1520-5. Available from: https://doi.org/10.1124/dmd.31.12.1520
- Goon D, Klaassen CD. Dose-dependent intestinal glucuronidation and sulfation of acetaminophen in the rat in situ. J Pharmacol Exp Ther. 1990;252(1):201-7. Available from: https://pubmed.ncbi.nlm.nih.gov/2299589/
- Gessner T, Hamada N. Identification of p-nitrophenyl glucoside as a urinary metabolite. J Pharm Sci. 1970;59(8):1528-9. Available from: https://doi.org/10.1002/jps.2600591043
- Quebbemann AJ, Anders MW. Renal tubular conjugation and excretion of p-nitrophenol in the chicken: differing mechanisms of the renal transfer. J Pharmacol Exp Ther. 1973;184(3):695-708. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0022356525294617
- Li C, Shang D, Wang Y, Li J, Han J, Wang S, et al. Characterizing the network of drugs and their affected metabolic subpathways. PLoS One. 2012;7(11):e47326. Available from: https://doi.org/10.1371/journal.pone.0047326
- Li X, Li C, Shang D, Li J, Han J, Miao Y. The implications of relationships between human diseases and metabolic subpathways. PLoS One. 2011;6(7):e21131. Available from: https://doi.org/10.1371/journal.pone.0021131
- Ueng YF, Don MJ, Peng HC, Wang SY, Wang JJ, Chen CF. Effects of Wu-chu-yu-tang and its component herbs on drug-metabolizing enzymes. Jpn J Pharmacol. 2002;89(3):267-73. Available from: https://doi.org/10.1254/jjp.89.267
- Dong RH, Fang ZZ, Zhu LL. Investigation of UDP-glucuronosyltransferases (UGTs) inhibitory properties of carvacrol. Phytother Res. 2012;26(1):86-90. Available from: https://doi.org/10.1002/ptr.3525
- McNamara KM, Nakamura Y, Miki Y, Sasano H. Phase two steroid metabolism and its roles in breast and prostate cancer patients. Front Endocrinol (Lausanne). 2013;4:116. Available from: https://doi.org/10.3389/fpsyt.2013.00116
- Jaggi R, Addison RS, King AR, Suthers BD, Dickinson RG. Conjugation of desmethylnaproxen in the rat: a novel acyl glucuronide-sulfate diconjugate as a major biliary metabolite. Drug Metab Dispos. 2002;30(2):161-6. Available from: https://doi.org/10.1124/dmd.30.2.161
- Kuehl GE, Lampe JW, Potter JD, Bigler J. Glucuronidation of nonsteroidal antiinflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos. 2005;33(7):1027-35. Available from: https://doi.org/10.1124/dmd.104.002527
- Salmela PI, Sotaniemi EA, Pelkonen RO. The evaluation of the drug-metabolizing capacity in patients with diabetes mellitus. Diabetes. 1980;29(11):788-94. Available from: https://doi.org/10.2337/diacare.20.10.788
- van de Kerkhof EG, de Graaf IA, de Jager MH, Groothuis GM. Induction of phase I and II drug metabolism in rat small intestine and colon in vitro. Drug Metab Dispos. 2007;35(6):898-907. Available from: https://doi.org/10.1124/dmd.106.014563
- Beetstra JB, van Engelen JG, Karels P, van der Hoek HJ, de Jong M. Thyroxine and 3,3',5-triiodothyronine are glucuronidated in rat liver by different uridine diphosphate-glucuronyltransferases. Endocrinology. 1991;128(2):741-6. Available from: https://doi.org/10.1210/endo-128-2-741
- Fenk CJ, Kaufman N, Gerbig DG. A new colorimetric assay of tabletop sweeteners using a modified biuret reagent. J Chem Educ. 2007;84(10):1676. Available from: http://dx.doi.org/10.1021/ed084p1676
- Almási A, Fischer E, Perjési P. A simple and rapid ion-pair HPLC method for simultaneous quantitation of 4-nitrophenol and its glucuronide and sulfate conjugates. J Biochem Biophys Methods. 2006;69(1-2):43-50. Available from: https://doi.org/10.1016/j.jbbm.2006.05.002
- Almási A, Fischer E, Perjési P. HPLC quantification of 4-nitrophenol and its conjugated metabolites from bile. Sci Pharm. 2011;79(4):837-47. Available from: https://doi.org/10.3797/scipharm.1106-22
- Fischer E, Almási A, Bojcsev S, Fischer T, Kovács NP, Perjési P. Effect of experimental diabetes and insulin replacement on intestinal metabolism and excretion of 4-nitrophenol in rats. Can J Physiol Pharmacol. 2015;93(6):459-64. Available from: https://doi.org/10.1139/cjpp-2015-0065
- Haughton CL, Dillehay DL, Phillips LS. Insulin replacement therapy for the rat model of streptozotocin-induced diabetes mellitus. Lab Anim Sci. 1999;49(6):639-44. Available from: https://pubmed.ncbi.nlm.nih.gov/10638500/
- Inoue H, Yokota H, Taniyama H, Kuwahara A, Ogawa H, Kato S. 1-Naphthol beta-D-glucuronide formed intraluminally in rat small intestine mucosa and absorbed into the colon. Life Sci. 1999;65(14):1579-88. Available from: https://doi.org/10.1016/s0024-3205(99)00403-8
- Kothare PA, Zimmerman CL. Intestinal metabolism: the role of enzyme localization in phenol metabolite kinetics. Drug Metab Dispos. 2002;30(5):586-94. Available from: https://doi.org/10.1124/dmd.30.5.586
- Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13(3):294-307. Available from: https://doi.org/10.1016/j.cmet.2011.01.018
- Zhao M, Yu S, Zhang M. Differential expression of multidrug resistance-related proteins in adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human bladder cancer cell lines. Mol Med Rep. 2016;14(5):4741-6. Available from: https://doi.org/10.3892/mmr.2016.5806
- Almasi A, Pinto EDLN, Kovacs NP. Changes in hepatic metabolic enzyme activities and biliary excretion of 4-nitrophenol in streptozotocin-induced diabetic rats. Braz J Pharm Sci. 2018;54:e17347. Available from: https://doi.org/10.1590/s2175-97902018000117347
- Vaswani R, Shukla S, Acharya S. Pathophysiology of complication in diabetes mellitus. J Pharm Res Int. 2021;33(60A):89-95. Available from: https://doi.org/10.9734/jpri/2021/v33i60A34459
- White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40 Suppl 2:S2-17. Available from: https://doi.org/10.1007/s001250051387
- Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). 2006;21:362-9. Available from: https://doi.org/10.1152/physiol.00024.2006
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
