Open Journal of Pharmacology and Pharmacotherapeutics
Potential Protective Effect of Cocoa Flavanols in Type 2 Diabetes: A Systematic Literature Review of Human Studies
Díaz López Cristina*
Pharmacy Faculty, Department of Biochemistry and Molecular Biology, University Complutense of Madrid, Spain
Cite this as
Cristina DL. Potential Protective Effect of Cocoa Flavanols in Type 2 Diabetes: A Systematic Literature Review of Human Studies. Open J Pharmacol Pharmacother. 2025;10(1):001-013. Available from: 10.17352/ojpp.000026Copyright License
© 2025 Cristina DL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Objective: This systematic review aims to critically evaluate existing human epidemiological and intervention studies investigating the potential protective effects of cocoa flavanols against Type 2 Diabetes Mellitus (T2DM).
Methods: A systematic literature search was conducted using PubMed, Science Direct, and Web of Science databases, covering the period of the last 15 years. Keywords used included “cocoa,” “flavan-3-ols,” “type 2 diabetes,” “interventional studies,” and “epidemiological studies.” Inclusion criteria involved original research articles examining cocoa flavanol intake and its effects on glycemic control, insulin sensitivity, lipid profile, endothelial function, and oxidative stress biomarkers in humans. Exclusion criteria included non-human studies, reviews, and studies lacking defined outcomes, and articles without clearly defined outcomes were excluded. PRISMA guidelines were followed for the review protocol.
Main findings: Eighteen studies were included: eight epidemiological and ten intervention studies. Epidemiological studies generally demonstrated an inverse association between moderate, regular cocoa consumption and risk of developing T2DM, particularly evident in populations with normal weight and healthy lifestyle practices. Intervention studies yielded mixed results: improvements in insulin sensitivity, endothelial function, lipid profiles, and oxidative stress were observed in some studies, especially with chronic, high-dose cocoa flavanol intake. However, other studies reported no significant effects, possibly due to methodological heterogeneity, short durations, and variability in flavanol dosages.
Conclusion: Although evidence from human studies suggests cocoa flavanols may have beneficial effects in managing or preventing T2DM, findings are not entirely consistent across studies. Well-controlled, long-term clinical trials with standardized flavanol dosages and clearly defined populations are necessary to firmly establish the clinical utility of cocoa flavanols for diabetes prevention or management.
Abbreviations
AP-1: Activator Protein 1; ATP: Adenosine Triphosphate; CAT: Catalase; CT: Total Cholesterol; DPP-4: Dipeptidyl Peptidase-4; DMT2: Type 2 Diabetes Mellitus; eNOS: Endothelial Nitric Oxide Synthase; FMD: Flow-Mediated Dilation; FFQ: Food Frequency Questionnaire; GIP: Gastric Inhibitory Polypeptide; GLP-1: Glucagon-Like Peptide-1; GLUT: Glucose Transporter; GTT: Glucose Tolerance Test; HbA1c: Glycated Hemoglobin; HDL: High-Density Lipoprotein; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; ICAM-1: Intercellular Adhesion Molecule-1; BMI: Body Mass Index; LDL: Low-Density Lipoprotein; MODY: Maturity Onset Diabetes of the Young; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; NF-κB: Nuclear Factor Kappa B; Nrf2: Nuclear Factor Erythroid 2-Related Factor 2; PSGL-1: P-Selectin Glycoprotein Ligand-1; QUICKI: Quantitative Insulin Sensitivity Check Index; ROS: Reactive Oxygen Species; SGLT: Sodium-Glucose Transporter; SOD2: Superoxide Dismutase 2; TG: Triglycerides; VLDL: Very Low-Density Lipoprotein
Introduction
The World Health Organization (WHO) defines diabetes as a “chronic disease that occurs when the pancreas does not produce enough insulin or when the body does not effectively use the insulin it produces” (WHO, 2020).
This condition represents a major public health concern due to a continuous increase in its prevalence and incidence worldwide, as well as its significant economic impact.
According to the latest data published by the International Diabetes Federation in 2019 [1], approximately 463 million people between the ages of 20 and 79 live with this disease, representing 9.3% of the population. If effective preventive measures are not implemented, this figure is estimated to increase to 578 million by 2030 (10.2%) and to 700 million by 2045 (10.9%). Furthermore, 11.3% of deaths from all possible causes in people aged 20 to 79 are due to diabetes. 79 years of life expectancy is caused by diabetes, affecting more women (2.3 million) than men (1.9 million) [1].
It is also worth mentioning the significant economic impact resulting from the prevention, diagnosis, and treatment of the disease, as well as its complications. Direct costs stem primarily from hospitalizations due to long-term complications, while indirect costs stem from comorbidity, disability, and premature mortality [1].
In Spain, the most recent data, published by the Center for Biomedical Research in Diabetes and Metabolic Diseases (CIBERDEM) and obtained from the second phase of the [email protected] study, establish an incidence of the disease at 11.6%, representing approximately 386,000 new cases each year [2]. Furthermore, the prevalence data published by the National Institute of Statistics show a different pattern than the rest of the world in terms of gender distribution, with women being more affected than men in all age groups [3].
In 2018, type 2 diabetes mellitus (T2DM) was the tenth most common cause of death in Spain, accounting for 2.3% of all deaths and affecting more women than men. It was also reported as a multiple cause (associated with other diseases) of death in 8.7% of cases [4].
The increase in the number of cases in both Spain and the rest of the world is due to multiple factors, which can be attributed to an aging population and Lifestyle changes including urbanization, physical inactivity, and poor dietary habits. [2] Environmental factors have a major influence, so leading a healthy lifestyle makes the disease potentially preventable. Diet is currently considered a key factor in preventing or delaying the development of the disease, in addition to being a low-cost measure. In this sense, identifying dietary components as potential antidiabetic agents has become a major research challenge [5].
There is a growing body of work supporting the antidiabetic properties of certain compounds present in the diet, primarily polyphenols, which can be used as a therapeutic and preventive strategy for T2DM. In vivo and in vitro studies have shown that these compounds can improve glucose homeostasis, exerting a hypoglycemic effect through different mechanisms of action in the intestine, liver, or pancreatic β cells [6]. The data available to date suggest benefits, but it remains unknown whether the consumption of foods rich in polyphenols can be recommended to the general population, so further studies are needed to confirm these findings.
Type 2 diabetes
Pathophysiology
Diabetes is a set of complex metabolic disorders that lead to prolonged hyperglycemia. There are four main types of diabetes: type 1 diabetes mellitus (T1DM), T2DM, gestational diabetes, and MODY (Maturity Onset Diabetes of the Young).
T2DM accounts for approximately 90% of all diabetes cases. Its onset is insidious, its development gradual, and it is often asymptomatic, generally being diagnosed during routine blood tests. It was once considered a disease characteristic of adults, but in recent years, its incidence in young people has progressively increased, linked to a rising prevalence of obesity, one of the most important risk factors for the development of this disease [7].
To understand the pathophysiology of diabetes, it is important to understand the role of insulin in the body. Insulin is synthesized by pancreatic β-cells and is released in response to elevated blood glucose levels following a biphasic pattern. [8].
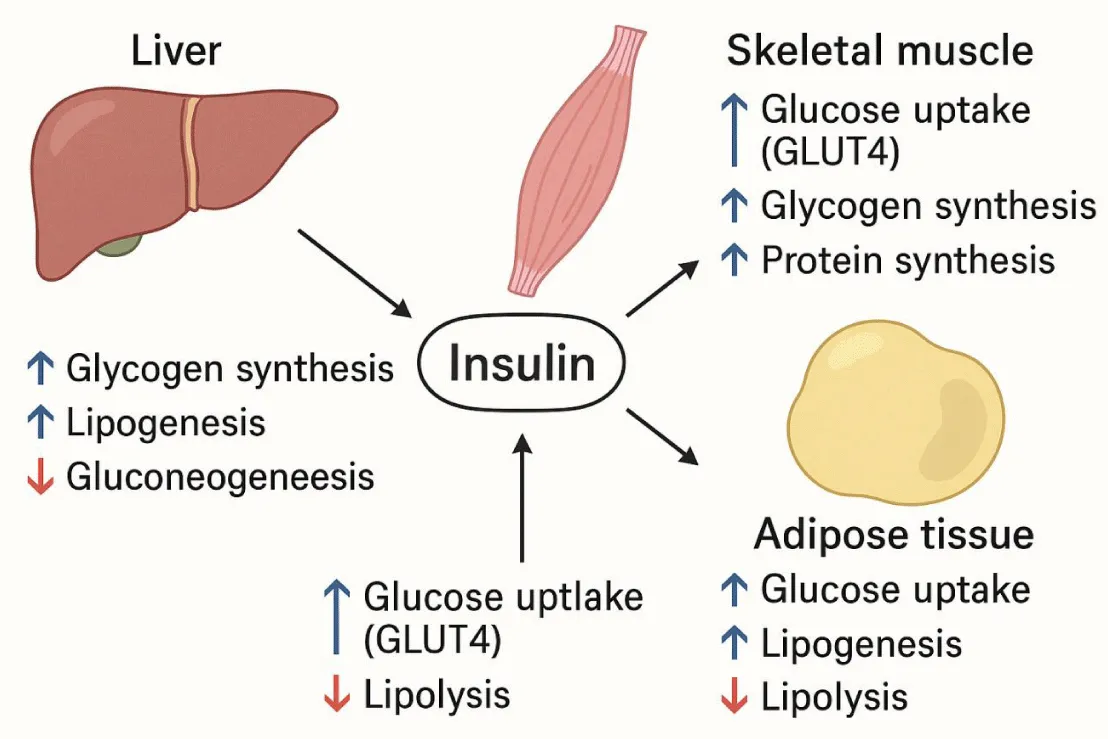
Once in the bloodstream, insulin exerts distinct anabolic actions in the liver, adipose tissue, and skeletal muscle (Table 1, Figure 1).
Insulin facilitates anabolic processes across multiple tissues: enhancing glycogen synthesis and lipogenesis in the liver, promoting glucose uptake and protein synthesis in skeletal muscle, and increasing lipogenesis while reducing lipolysis in adipose tissue.
The development of T2DM results from the body’s inability to maintain adequate blood glucose levels. This is due to a defect in the insulin-secretory response to glucose and/or a decrease in tissue sensitivity to the effects of this hormone in muscle, liver, and adipose tissue. This leads to a decrease in insulin-induced glucose transport to adipocytes and skeletal muscle, increased glucose production in the liver, and alterations in lipid metabolism in adipose tissue and liver [8].
During the development of insulin resistance, β cells initially maintain normal glucose tolerance, but are constantly stimulated, resulting in increased insulin production (hyperinsulinemia). At a given moment, the released levels cannot compensate for this resistance, triggering the sustained hyperglycemia that characterizes diabetes and, in the long term, the insufficiency and dysfunction of β cells [9].
Insulin resistance also contributes to alterations in lipid metabolism. It stimulates lipogenesis and leads to inadequate fatty acid oxidation. Levels of free fatty acids, which act as a substrate for triglyceride synthesis in the liver, are elevated, potentially leading to hypertriglyceridemia. Production of Very Low-density Lipoproteins (VLDL) increases, and levels of High-density Lipoproteins (HDL) decrease [10].
Hyperglycemia and hyperlipidemia trigger toxicity processes (glucotoxicity and lipotoxicity), which are associated with increased levels of Reactive Oxygen Species (ROS) and, therefore, oxidative stress. Excessive generation of these ROS is linked to the development of long-term complications of diabetes, in addition to contributing to a decrease in the number and function of β cells, which are inherently more sensitive to oxidative stress due to their low levels of antioxidant enzymes. [11].
Among the complications of diabetes are, on the one hand, macrovascular complications, which can lead to stroke, coronary artery disease, angina, heart failure, and peripheral vascular disease; these are associated with ulcer formation and lower extremity amputation. On the other hand, there are microvascular complications, which include retinopathy, which can cause blindness; nephropathy, which can lead to kidney failure; and neuropathy, which damages nerves and causes weakness and loss of sensation in the extremities [8].
Taken together, all these complications of diabetes significantly affect patients’ quality of life and constitute the leading cause of disability and premature mortality in most developed countries.
Dietary flavanols
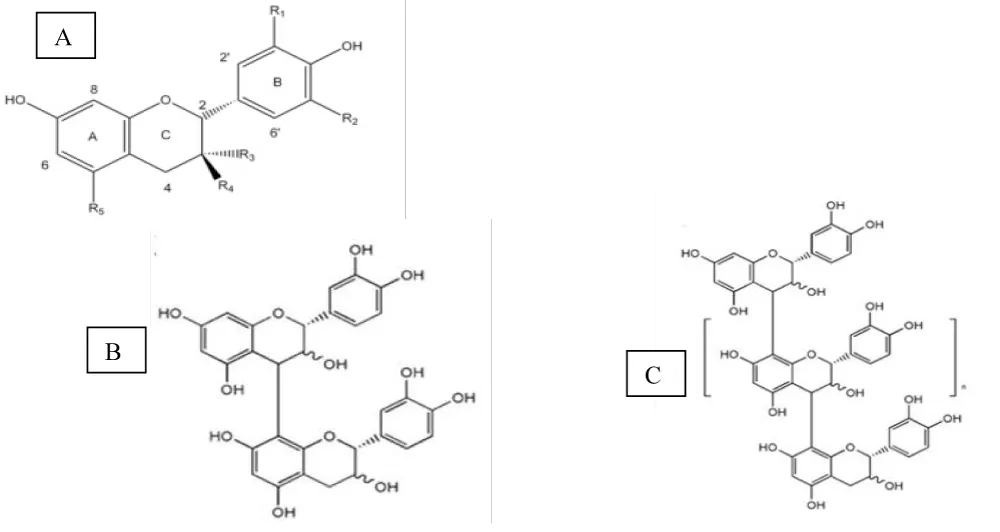
Polyphenols are secondary metabolites present in plants and constitute the largest group of phytochemicals within the plant kingdom. Among them, flavonoids are considered the most important polyphenols, representing almost 60% of the polyphenols we consume in our diet. Their basic skeleton (C6-C3-C6) consists of two aromatic rings (A and B) linked by a three-carbon chain, generally organized as an oxygenated heterocycle (ring C). They are divided into different subclasses based on the nature of the heterocyclic ring and the substituents they contain: flavanols, flavonols, flavones, flavanones, isoflavones, and anthocyanins. Flavanols possess a hydroxyl group at the C3 position of the heterocyclic ring and are also called flavan-3-ols. They exist as monomers, called catechins, and as oligomers or polymers (with varying degrees of polymerization), called proanthocyanidins. Unlike other flavonoids, flavanols typically occur in non-glycosylated form in foods [11].
The main flavanol monomers (Figure 2) appear in foods such as cherries, plums, blackberries or apples, in the form of (+)-catechin and (-)-epicatechin; while (-)-epigallocatechin gallate, (-)-epigallocatechin and (-)-epicatechin gallate are found in some legume seeds such as beans, in grapes and especially in green tea.
Although flavanols are found in significant levels in fruits such as apples, berries, plums, apricots, and nuts, cocoa, grapes, and tea are the foods par excellence with the highest content of these compounds. Their concentration can vary due to factors such as harvest time, plant variety, environmental factors, processing, and storage conditions [5] For example, catechins are found in fruits such as cherries and apricots at concentrations of up to 250 mg/kg and in beverages such as red wine at up to 300 mg/L, although the main sources are cocoa (up to 600 mg/L) and green tea (up to 800 mg/L). Proanthocyanidins are the flavanols responsible for the astringent character of some fruits and beverages, as well as the bitterness of chocolate. Their quantity in food is difficult to determine due to the complexity of their chemical structure [11]. The chemical structure of flavanols and the food matrix determine their bioavailability and absorption in the body.
Dietary flavanols have numerous beneficial actions (antioxidant, anti-inflammatory, and antidiabetic, among others), and several general mechanisms have been proposed to explain this bioactivity:
- Antioxidant and metal sequestration: The presence of phenolic hydroxyl groups in these compounds makes them capable of neutralizing free radicals by donating an electron and stabilizing them through resonance via their aromatic chemical structure. The antioxidant potential depends on the arrangement and number of phenolic hydroxyl groups present. They act directly, preventing the generation of ROS by breaking the cycle of new free radical generation and chelating metal ions involved in free radical production. They also act indirectly, regulating the activity of antioxidant enzymes (superoxide dismutase, catalase, glutathione synthase) and pro-oxidant enzymes (cyclooxygenase, nitric oxide synthase). On the other hand, they regulate the activity of transcription factors such as Nrf2 or NF-κB, which are important in the response to oxidative stress [12].
- Interaction with cell membranes: The presence of hydrophobic and hydrophilic domains in their structure allows them to position themselves on the bilayer surface adsorbed on the polar head of lipids and/or to insert into the bilayer and interact with the hydrophobic chains of lipids. This depends on the degree of polymerization and the number and distribution of hydroxyl groups. Thus, flavanols could affect cellular function by modifying the structure of the plasma membrane and its physical characteristics, such as fluidity and electrical properties [12].
- Interaction with enzymes: They primarily act by exerting inhibitory actions on numerous enzymes. These include those that have purines as substrates (e.g., DNA polymerase or xanthine oxidase) and enzymes that have NADPH as a cofactor (e.g., aldose reductase or nitric oxide synthase). This is possible through competitive binding to the NADPH binding site on the enzyme. It can be hypothesized that certain polyphenols act by inhibiting ATP-dependent enzymes, through a competitive mechanism for the ATP binding site on the enzyme. This competition appears to depend on the presence of two hydroxyl group substitutions at position 5,7 of the A ring and a 2,3-unsaturation along with a 4-keto group in the C ring [13].
- Interaction with transcription factors: Among polyphenols, certain flavanols (such as epicatechin, (+)-catechin, or certain procyanidins) can modulate the expression of numerous AP-1 or NF-κB-regulated genes involved in inflammation and carcinogenesis. In these cases, not only the chemical composition is important, but also the spatial conformation of the molecule, as this determines whether or not these specific interactions occur [12].
Cocoa as a source of flavanols
Cocoa and its derivatives are widely consumed worldwide. It is a food rich in protein (15-20%), carbohydrates (15%), fiber (26-40%), lipids (20-24%), minerals (Ca, K, Mg), and vitamins (A, B, and E) [11].
Cocoa is widely recognized as a rich source of dietary antioxidants due to its high flavonoid content, primarily flavan-3-ols and procyanidins, as well as flavonols (quercetin, isoquercetin, and quercetin-3-O-arabinoside) and anthocyanins. Total polyphenol content accounts for 12–18% of the seed’s dry weight, of which procyanidins, catechins, and anthocyanins are the most abundant, accounting for 58%, 37%, and 4%, respectively, of total polyphenols. The main procyanidins present in cocoa are procyanidins B1 and B2, although it also contains procyanidins B3, B4, B5, C1, and D. The main monomeric flavanol is (-)-epicatechin, accounting for up to 35% of the total catechins, along with (+)-catechin, (+)-gallocatechin, and (-)-epigallocatechin. Cyanidin-3-α-L-arabinose and cyanidin-3-β-D-galactose are the main anthocyanins in cocoa [5].
Other polyphenolic derivatives, such as phenolic acids, flavones (luteolin and apigenin), flavanones (narigenin), stilbenes, simple phenols, and iso-coumarins, are also present in cocoa, although in smaller proportions.
Regarding methylxanthines, cocoa beans contain between 1 and 4% theobromine and 0.2-0.5% caffeine, while theophylline is found in very small concentrations and only in some varieties. These compounds may also have a beneficial effect, increasing HDL cholesterol levels and reducing LDL cholesterol levels [5].
In addition to the antioxidant, anti-inflammatory, and hypolipidemic effects demonstrated by cocoa flavanols, many publications have emphasized their potential antidiabetic activity. Therefore, these compounds can be good chemopreventive agents against the development of diabetes since they regulate the digestion and absorption of carbohydrates, promote the uptake of glucose in skeletal muscle and adipose tissue, improve insulin secretion, promote the proliferation of β cells and reduce their apoptosis [14,15].
The most direct mechanism by which flavanols can improve glucose homeostasis is by slowing the digestion and absorption of carbohydrates in the intestine. Flavanols can inhibit α-amylase and α-glucosidase, disaccharidases that transform carbohydrates into simpler molecules, through non-covalent bonds via their hydroxyl groups. Here, the total flavanol concentration and structural complexity play an important role in the inhibitory capacity. Dipeptidyl peptidase-4 (DPP-4) is also inhibited, thereby improving the response to incretins (GLP-1 and GIP), although the mechanisms are not exactly known. Cocoa polyphenols also exert this inhibitory action on enzymes that act in the pancreas, such as phospholipase A2, pancreatic lipase, and α-amylase. They also act on glucose transporters, such as GLUT2 and SGLT1, through a competitive inhibition mechanism at the active site of transport or through nonspecific flavanol-transporter interactions. This occurs primarily in the intestine, as bioavailability is low and the metabolites have a short half-life. The overall effect involves a decrease in macronutrient absorption and energy intake [16].
The regulation of glucose homeostasis, as well as insulin secretion, is mediated through the participation of flavanol metabolites in multiple cell signaling-pathways in the pancreas, liver, skeletal muscle, and white adipose tissue. These mechanisms are highly specific, sometimes even mediated by a single class of flavanols [15].
As previously mentioned, one of the consequences of T2DM is β-cell deterioration. Studies in animals and cell cultures support the idea that flavan-3-ols improve β-cell viability, increasing their mass and functionality by increasing the expression of certain components of the insulin signaling cascade. Furthermore, due to their antioxidant action, cocoa flavanols can protect β-cells from oxidative damage by acting as ROS scavengers and improving insulin secretion, in addition to inducing their proliferation. They also exert protective effects by inhibiting lipid accumulation in β cells, thereby reducing lipotoxicity derived from chronic hyperlipidemia that occurs in diabetes [15].
Finally, it is worth noting that decreased insulin sensitivity worsens endothelial function, leading to the long-term development of macrovascular complications. Physiologically, insulin increases blood flow in skeletal muscle and thus vasodilation-mediated glucose absorption. When insulin resistance occurs, the activity of the enzyme nitric oxide synthase (eNOS) in the vascular endothelium is reduced, and with it, nitric oxide production. Oral administration of flavan-3-ols produces a hypotensive effect, inducing eNOS expression and, consequently, increased nitric oxide generation, improving flow-mediated dilation. This, in addition to improving endothelial function, indirectly promotes glucose uptake [17].
All these effects make cocoa a highly valuable food for preventive or therapeutic use in diabetes alone or in combination with other polyphenol-rich foods and/or medications. Therefore, the main objective of this paper is to review the various epidemiological and intervention studies conducted in humans to date on the effects of cocoa on subjects with T2DM, which may or may not support this potential preventive and/or therapeutic effect.
Among dietary components studied for their potential antidiabetic benefits, polyphenols—particularly flavanols abundant in cocoa—have shown significant promise. Cocoa flavanols possess antioxidant, anti-inflammatory, and insulin-sensitizing properties, making them candidates for preventive and therapeutic dietary strategies in managing type 2 diabetes [11,16]. Despite substantial evidence from animal and cellular studies highlighting their beneficial effects on glucose homeostasis, insulin sensitivity, and pancreatic β-cell function, human studies have produced varied results. Thus, systematically assessing the evidence from human epidemiological and intervention studies becomes essential to clearly define the potential clinical value of cocoa flavanols in diabetes management.
Materials and methods
Systematic literature research
For this paper, a systematic bibliographic search of the existing scientific literature was conducted, from which various documents were selected, including scientific articles and textbooks.
To locate and select the bibliography and to cover the maximum available and up-to-date information on the chosen topic, specific health sciences documentary sources were used:
Databases: Science Direct, PubMed, and Web of Science. The objective was to identify peer-reviewed scientific papers published in the past 15 years and available in full-text format and that investigated the potential beneficial effects of dietary chocolate or cocoa consumption in healthy subjects and those with T2DM. The search used keywords such as “cocoa” AND “flavan-3-ols” AND “type 2 diabetes” AND “interventional studies” OR “epidemiologic studies.” Additionally, relevant meta-analyses and systematic reviews were consulted to support the evidence base. To assess the relevance of the articles to the study topic, the abstracts were sufficient, although in some cases it was necessary to review the full articles.
Public Institutions Archives: National Institute of Statistics (INE), International Diabetes Federation (IDF), Ministry of Health, Consumer Affairs, and Social Welfare (MSCBS), and World Health Organization (WHO).
Textbooks: Textbooks on pathophysiology and biochemistry that provided extensive information on diabetes from a pathophysiological and biochemical perspective, as well as the actions of insulin in the body, were used.
Biochemical biomarkers
The main parameters and biochemical markers present in the different studies (Figure 3), given that they are the most commonly used for the diagnosis and monitoring of T2DM and, therefore, considered in this study, are:
- Body mass index (BMI)
- Blood glucose, insulin, and glycated hemoglobin (HbA1c).
- Glucose tolerance test (GTT)
- Insulin resistance assessment models: QUICKI (Quantitative Insulin Check Index) and HOMA-IR (Homeostasis Model Assessment).
- Vascular function: blood pressure (BP), endothelial adhesion molecules (ICAM-1, PSGL-1, E-selectin), and flow-mediated dilation (FMD).
- Oxidative stress: 15-F2t-isoprostane, glutathione, catalase (CAT), and superoxide dismutase type 2 (SOD2).
- Lipid profile: total cholesterol (TC), HDL cholesterol, LDL cholesterol, and triglycerides (TG).
The figure summarizes the flow of information through the different phases of the review. It shows the number of records identified, included, and excluded, and the reasons for exclusions at each stage.
Results
Eighteen scientific studies were identified that examined the effect of cocoa intake on people with T2DM over the past 15 years (from 2005 to 2020). Of these, eight were epidemiological studies and ten were intervention studies.
Description of the epidemiological studies
Eight epidemiological studies were included, aimed at evaluating the association between varying levels of chocolate consumption and the risk of developing T2DM of chocolate (as a source of cocoa) may influence the risk of developing T2DM.
All studies selected a cohort of individuals under well-defined inclusion/exclusion criteria and conducted prospective follow-up to assess the incidence of diabetes in the study population. Some studied healthy subjects and others studied subjects at cardiovascular risk, as in the study by Greenberg, et al. [18].
In all of them, dietary chocolate consumption is assessed using validated, study-specific food frequency questionnaires. One of the most important variables that allows for the classification of individuals across studies is the frequency of chocolate consumption, which can be weekly or monthly (e.g., less than once a month, more than once a week, etc.). Based on the collected results, many studies estimate polyphenol and even flavanol consumption using Phenol Explorer, a database that displays flavanol concentrations in different foods. Other factors such as comorbidities, lifestyle, and education are also taken into account. This can be seen in secondary analyses conducted by some studies, such as that of Matsumoto, et al. [19].
Main results of the epidemiological studies
The epidemiological studies reviewed and their main results are summarized in Tables 2,3. The study by Matsumoto, et al. [19] selected a cohort of 18,235 diabetes-free men aged 57 to 75 years. Chocolate consumption was monitored using a Food Frequency Questionnaire (FFQ). Chocolate consumption was classified into four categories: never or rarely consumed chocolate, 1-3 servings/month, 1-2 servings/week, and more than 2 servings/week. Other variables such as alcohol consumption, smoking, exercise, and BMI were also taken into account. After a mean of 9.2 years of follow-up, the incidence of diabetes was 6.2%. In secondary analyses, the inverse association between chocolate consumption and the risk of T2DM was greater in subjects with no history of cardiovascular disease. In this prospective study, chocolate intake was significantly and inversely associated with T2DM, and this relationship appeared to be limited to normal body weight and younger men after adjustment for lifestyle, clinical, and dietary risk factors.
Zamora-Ros, et al. [20] selected a cohort of 340,234 individuals from eight European countries to evaluate the association between individual flavanol intake and the development of T2DM. Consumption of chocolate and other flavonoid-rich foods was measured with country-specific FFQs. Other variables such as physical activity and smoking were included. During 17 years of follow-up, 12,403 individuals developed diabetes (incidence 3.6%). This large, heterogeneous, prospective study supports a protective role for various flavan-3-ol monomers and low-degree polymerization proanthocyanidins against T2DM, in addition to the different roles that individual flavonoids may play in the etiology of the disease.
In their study, Oba, et al. [21] examined the risk of diabetes in a Japanese population in relation to the consumption of a range of foods and beverages such as coffee, tea, and chocolate over a 10-year follow-up period. This was measured using a semi-quantitative FFQ in a cohort of 5,897 men and 7,643 women. To assess the association, age, smoking, BMI, physical activity, alcohol consumption, caloric intake, fat intake, and menopausal status of women were adjusted for. A 35% reduction in the risk of diabetes was observed in men who consumed chocolate at least once per week, compared to those who never or rarely ate chocolate. Similar associations were found in women. This suggests that the lowest risk may be observed among moderate chocolate consumers (at least once per week).
Crichton, et al. [22] analyzed a cohort of 953 individuals with a mean age of 62 to assess the beneficial effects of regular chocolate intake on T2DM over a 30-year follow-up period. Chocolate intake was assessed using a FFQ, dividing individuals based on the number of times per week they ate chocolate. It was found that T2DM prevalence was notably lower among individuals who consumed chocolate more than once per week than in those who never or rarely ate chocolate. These results were observed after adjustment for cardiovascular, lifestyle, and dietary factors, including other polyphenol-rich beverages such as coffee or red wine.
In the study by Maskarinek, et al. [23], a cohort of 151,691 individuals aged 45–75 years of different ethnicities was observed for an average of 8 years, with an incidence of T2DM cases of 5.6% (8,487 people). Dietary intake was assessed using a validated FFQ. It was found that the daily and frequent intake of chocolate (in sweets and beverages) in a normal diet was inversely related to the risk of developing T2DM, with statistically significant differences seen in older individuals compared to younger individuals and in Japanese Americans compared to other ethnicities, where there were hardly any significant differences. This inverse association was also restricted to individuals with normal weight and without comorbidities at entry into the cohort.
Greenberg, et al. [24] conducted a study in the United States to explain the possible association between long-term chocolate consumption habits and the risk of diabetes, based on the hypothesis that the results would be similar to the study by Oba, et al. [21] discussed above. A cohort of 15,732 people aged between 45 and 64 years was followed for an average of 13.3 years. The incidence of diabetes was 5.4%. More frequent chocolate consumption was associated with a lower risk of diabetes, up to 2–6 one-ounce servings per week. However, there was no significant association for consumption of more than or equal to one serving per day. There were also no significant differences between men and women.
Finally, a subsequent study was conducted on this same group [18], this time selecting a cohort of 92,678 postmenopausal women. An inverse association was found between the frequency of chocolate consumption and the risk of T2DM during a 13-year follow-up. There were 10,804 cases of T2DM during the study, representing an incidence of 11.7%. There was no significant linear association between long-term chocolate intake and the risk of T2DM, but there was a significantly reduced risk at moderate levels of intake. This finding was robust, as it did not change after excluding women who had suffered a myocardial infarction, stroke, cancer or other conditions. Other secondary analyses associated a lower risk of T2DM in women who were less physically active than average and over 65 years of age. Regarding BMI, no statistically significant results were obtained (Table 2).
Description of human intervention studies
A total of 10 human studies were included in the search. Those that evaluated the effect of cocoa on subjects with T2DM and other conditions such as hypertension were selected. In one study, the study subjects were postmenopausal women, which is associated with increased cardiovascular risk.
With the exception of two pilot studies, the rest are randomized, double-blind, placebo-controlled clinical trials. The objective of using a control group was to determine whether there was sufficient statistical evidence that the beneficial effects obtained were due to cocoa consumption and no other variables. Depending on the study, the control group received no cocoa or a low-polyphenol cocoa source.
Supplementation was generally given in the form of a cocoa drink, high-percentage dark chocolate, or enriched chocolates. Almost all the types of cocoa used have a high flavanol content, with the aim of being able to evaluate the action of cocoa at doses higher than or similar to those estimated to be ingestible in a normal diet and which, in addition, can produce significant effects on the analysis parameters.
The effects of (-)-epicatechin are generally studied, as it is the most abundant and clinically relevant flavan-3-ol among cocoa polyphenols, although some studies evaluate the action of polyphenols or flavanols as a whole.
With the exception of three studies that evaluated the acute effects after administration of a single dose of cocoa, the remaining studies involved daily supplementation, administered in one or multiple doses to evaluate long-term effects. The duration of these studies ranged from up to 6 hours in acute studies to 30 days to 1 year in chronic studies.
In general, the parameters analyzed in the different studies were: insulin resistance (via HOMA-IR), insulin sensitivity (QUICKI), endothelial function (FMD, ICAM, E-selectin, PSGL-1), glycemia (fasting and postprandial), HbA1c, BMI, blood pressure, oxidative stress (15-F2t -isoprostane, glutathione, CAT, SOD2) or lipid metabolism (TC, HDL, LDL, TG).
Main results of human intervention studies
The first four studies mentioned are studies on the effect of acute cocoa supplementation (Table 4). The patients were treated with antidiabetic, lipid-lowering, or antihypertensive drugs; therefore, the effect of flavanols was not evaluated alone but in combination with the medication.
In the study by Basu, et al. [25], the influence of consuming cocoa beverages containing 960 mg of polyphenols was studied in obese type 2 diabetic subjects after a high-fat breakfast. Samples were taken at 0.5, 1, 2, 4, and 6 hours postprandially. After 1 and 4 hours, an increase in HDL cholesterol was observed compared to placebo, but there was no effect on glucose, LDL, TG, and total cholesterol. On the other hand, an increase in insulin concentrations was observed at 4 hours, but no general effects on insulin resistance were seen.
In the second study [26], the effect of consuming a flavanol-rich cocoa powder with a diabetic-friendly breakfast was studied in patients medicated with T2DM. Each individual’s nutritional status was assessed before the trial. A 2.5 g cocoa powder was administered, and blood samples were taken before intake, and at 2 and 4 h postprandial. No significant effect was detected on blood glucose, TG, total cholesterol, HDL, LDL, and blood pressure levels. However, significant increases were observed for insulin and HOMA-IR at 2 h postprandial.
In their study, Mellor, et al. [27] investigated the effects of high-polyphenol chocolate on endothelial function and oxidative stress in obese subjects with T2DM following the administration of 75 g of glucose. Blood and urine samples were taken fasting and 2 h postprandially. No significant differences were observed in the study parameters after fasting (glucose, insulin, endothelial function, and oxidative stress), but differences were observed after the oral Glucose Tolerance Test (GTT). Oxidative stress was significantly reduced (lower urinary 15-F2t-isoprostane levels) and endothelial function improved in subjects who had consumed high-polyphenol chocolate, due to reduced levels of ICAM-1, E-selectin, and P-selectin glycoprotein ligand-1 (PSGL-1).
In the last study [28], 10 non-smoking subjects aged 50–80 years with T2DM were selected to conduct a cocoa bioavailability study. Flow-mediated dilation (FMD) of the brachial artery and plasma levels of flavanol metabolites were assessed after ingestion of a single cocoa beverage with increasing flavanol concentrations. These parameters were measured before and up to 6 hours afterward. Dose-dependent effects were observed, with significant acute increases in circulating flavanol metabolites and FMD at 2 hours. It was concluded that the effects of flavanols on FMD were positive for a mean of 4 hours in all subjects who consumed beverages containing 371 and 936 mg of flavanols, with no significant differences observed in the control group (75 mg).
The following seven studies (Table 4) show the long-term effects of cocoa consumption in subjects with T2DM and hypertension.
In the same study by Balzer, et al. [28], a study was also conducted with 41 subjects on the efficacy of long-term cocoa consumption. All participants randomly consumed a control cocoa drink (75 mg flavanols), a moderate-cocoa drink (371 mg flavanols), and a high-cocoa drink (963 mg flavanols). The main findings were that flavanols are absorbed in a dose-dependent manner and that daily cocoa intake improves arterial dilation, measured as FMD. However, there were no statistically significant differences in blood glucose, HbA1c, lipid profile, or blood pressure compared to the control group.
As in the previous study, Dicks, et al. [29] observed no significant differences in glycemic parameters, lipid profile, and blood pressure. Forty-two subjects with T2DM and hypertension who had good pharmacological and/or dietary control were selected. Each subject was administered 2.5 g of cocoa powder (ACTICOA™ brand), containing 207.5 mg of flavanols. After follow-up, no significant differences could be detected in blood pressure, glucose metabolism (fasting glucose, insulin, HbA1c, HOMA-IR), and lipid profile (TC, LDL, HDL, TG) between the study groups.
In the study by Mellor, et al. 30], the same results were obtained as in the previous two studies, except for HDL levels. Twelve diabetic subjects with good pharmacological control of their disease were selected. They were given 45 g of chocolate with or without high polyphenol content. The most notable result was an increase in HDL levels in the subjects who had consumed chocolate with high polyphenol content, which led to an improvement in the TC/HDL ratio. No changes were observed with the low-polyphenol chocolate in any parameter. Over the course of 16 weeks of daily chocolate consumption, neither weight nor glycemic control changed from baseline.
Ramírez-Sánchez, et al. [31] studied the influence of (-)-epicatechin on oxidative stress in subjects with T2DM and heart failure. After three months of intervention, there were no statistically significant differences in blood glucose or insulin levels. Treatment with (-)-epicatechin induced the recovery of mitochondrial structure, glutathione levels, increased expression of antioxidant enzymes such as SOD2 and catalase (CAT), in addition to decreased nitrotyrosylation and carbonylation of proteins.
On the other hand, the study by Curtis, et al. [32] evaluated whether the chronic intake of flavan-3-ols and isoflavones in postmenopausal women with T2DM controlled with pharmacological treatment could have positive effects on reducing cardiovascular risk. The 93 participants were administered 27 g of chocolate enriched with flavonoids (90 mg of epicatechin) and 100 mg of isoflavones. Compared with the placebo group, a significant reduction in peripheral insulin resistance (estimated with the HOMA-IR index) and an improvement in insulin sensitivity (determined with the QUICKI index) were observed as a result of a decrease in insulin levels. No effect was observed on blood pressure, HbA1c or glucose, but significant reductions were observed in total cholesterol, LDL and an improvement in the HDL-cholesterol ratio. The estimated 10-year risk of total coronary artery disease was attenuated after the flavonoid intervention.
Rostami, et al. [33] examined the effect of white and dark chocolate with a high cocoa content (83%) on lipid profile, body weight, blood pressure, glycemic control, and inflammation in 60 nonsmoking subjects aged 35–70 years with T2DM and hypertension controlled with medication. Twenty-five g (450 mg of flavonoids) of chocolate were administered for eight weeks. Consumption of dark chocolate with a high polyphenol content resulted in a significant decrease in blood pressure and fasting blood glucose compared to white chocolate. However, no significant effect of dark chocolate consumption was observed on fasting insulin, HbA1c, TG, LDL, and HDL levels.
In the study by Hagighat, et al. [34], the effect of dark chocolate on glycemic control and blood pressure was evaluated in diabetic and hypertensive patients. Twenty-five mg of dark chocolate containing 450 mg of flavonoids was administered. A study of 69 diabetic and hypertensive patients was conducted for eight weeks. They were randomly assigned to two groups: one to receive dark chocolate and the other to receive white chocolate. The intervention group had significantly lower blood pressure values, as well as lower fasting blood glucose and HbA1c levels, compared to the control group.
Discussion
In the epidemiological studies reviewed, an inverse relationship is established between chocolate consumption and the development of T2DM. The large sample size and follow-up time are the main advantages of this type of study. In all of them, individuals are grouped according to their chocolate consumption level, allowing for a better analysis of which intake levels show greater or lesser effects.
However, the findings regarding total cocoa polyphenol intake remain inconsistent, as the assessment of cocoa consumption in individuals is done through a validated questionnaire that records what is ingested in their regular diet (for example, through sweets or beverages). The type of chocolate consumed and, therefore, its cocoa content is not specified. While dark chocolate may confer health benefits, the flavanol content in milk chocolate is generally lower, given its lower concentration of cocoa flavanols and higher sugar content. Another limitation lies in the self-reporting of chocolate consumption by subjects, which may lead to errors when completing the questionnaires. Some studies suggest that rigorous placebos, rather than just assessing consumption levels, would be necessary to determine whether the effects obtained are truly derived from cocoa consumption [24]. Furthermore, the effects of cocoa polyphenols in the diet cannot be accurately assessed without considering other sources of these compounds (tea, grapes, plums, apricots, berries, etc.).
Working with a large sample size makes it difficult to narrow down the study variables for a more in-depth analysis of cocoa consumption in a given population. For example, in the multiethnic cohort study [23], chocolate consumption is assessed collectively in people who likely have very different nutritional patterns, so many variables (sex, lifestyle, ethnicity, weight, etc.) would have to be taken into account, making it difficult to control. Another study suggests that variables such as healthy lifestyle, which is also important for the development of diabetes, are not taken into account [20].
Although some studies point to a strong verification system for incident cases of T2DM as a strength [20,23], others point to it as a limitation [24]. This means that actual data on diabetes incidence are not being reported, resulting in over- or underestimation of the results.
For all the above reasons, these studies can serve as a starting point for an a priori evaluation; however, the results obtained need to be confirmed with intervention studies that do use defined doses of cocoa, which are known to contain sufficient amounts of polyphenols to achieve these potential beneficial effects.
Analyzing the selected intervention studies, there is notable heterogeneity among the findings. It should be noted that the main limitations of this type of research are the small sample size, the use of different types of cocoa as a source of polyphenols (beverages, dark chocolate, commercial preparations, etc.), and the short follow-up period. Fundamentally, changes have been found in parameters related to glucose homeostasis, endothelial function, lipid profile and oxidative stress.
Several in vitro and animal model studies have shown that cocoa flavanols can act as potential antidiabetic agents by improving insulin secretion and enhancing insulin sensitivity in peripheral tissues such as the liver, adipose tissue, and skeletal muscle [9]. Specifically, cocoa flavanols can improve insulin sensitivity by regulating glucose transport and key proteins in the insulin signaling pathway [14,16]. However, in the reviewed studies, significant reductions in fasting blood glucose were only found in two of the studies [33,34]; both after 8 weeks of intervention. On the other hand, significant variations in glycosylated hemoglobin were only observed in the study by Haghighat, et al. [33]. In the work of Rynarzewski, et al. [26], positive changes in HOMA-IR and insulinemia were observed after a prolonged period of chocolate consumption, regardless of the variety used; this did not occur in the study by Basu, et al. [25], detecting a significant increase in both insulin and HOMA-IR values 4 h after cocoa intake compared to placebo. It should be noted that this study consistently used the same type of food (a high-fat, diabetic-friendly breakfast), the sample size was small, and there was no control group; the authors argue that further studies are needed. Despite the high dropout rate and the good pharmacological control of the participants, the study by Curtis, et al. [32] showed a reduction in insulin resistance (HOMA-IR) and lower insulin levels after one year of follow-up. The limitations here are centered on the added use of isoflavones and the exclusive work with postmenopausal women. Therefore, it would be necessary to evaluate the effect of flavanols in isolation and determine whether these results are reproducible in other groups. In the remaining studies, no significant differences were observed in glucose metabolism parameters. This may be due to the use of subjects on multiple medications, as noted in the study by Dicks, et al. [29], since the active ingredients of antidiabetic, antihypertensive, and lipid-lowering drugs modulate targets common to those of flavanols. Similarly, it can be considered that the work is being carried out with insufficient doses due to the low bioavailability and the use of nutritional (non-pharmacological) quantities based on data from population studies.
The protective effect of polyphenols is thought to be due, in part, to their antioxidant action [12]. In the study by Ramírez-Sánchez, et al. (2013), an improvement in tredox status compared to the control was shown, with a recovery of glutathione levels in skeletal muscle and an increase in the expression of SOD22 and CAT; mitochondrial damage was also reduced. However, the pilot study had 5 subjects and the levels of (-)-epicatechin in the muscle could not be determined, so the results obtained could be due to other bioactive components of cocoa [31]. In the study by Mellor, et al. [30], similar results were also obtained, but this time through a decrease in 15-F2t-isoprostane in urine. Both studies used pure epicatechin, so this may provide insight into how this flavanol can reduce short- and long-term oxidative stress in future trials. The protective effects of cocoa on the development of diabetes can also be seen in animal models, such as the study by Fernández-Millán, et al. [35]. The findings of this study provide in vivo evidence of the protective effect of a cocoa-rich diet in prediabetic mice, as a delay in the loss of functional β-cell mass was observed, resulting from reduced oxidative stress and decreased apoptosis of these cells. This is supported by studies demonstrating the involvement of these compounds in metal sequestration, free radical scavenging, and interaction with antioxidant and pro-oxidant enzymes [12,16,36].
The benefits of cocoa on vascular endothelial function have been demonstrated in a substantial number of clinical trials. Its role as vasodilatory and hypotensive agents is particularly noteworthy, as they reduce arterial pressure and stiffness [37]. Some of the studies reviewed used hypertensive subjects, with beneficial responses seen in blood pressure parameters [33,34]. In the study by Balzer, et al. [28], the findings obtained support a significant improvement in endothelial function in a dose-dependent manner after the occasional intake of flavanol-rich cocoa, although subjects who took the control drink (75 mg of flavonoids) did not obtain significant benefits. The most striking improvement in FMD occurred at the same time as the highest levels of flavanol metabolites in plasma were reached (2 h). These results were also seen in the long-term trial. In the pilot study by Mellor, et al. [27], despite having a small sample size (n = 5), pronounced effects on the improvement of endothelial function were observed after subjecting the subjects to a state of transient hyperglycemia. This beneficial effect was attributed to increased nitric oxide production and a decrease in the expression of adhesion molecules such as ICAM-1 or P-selectin. Therefore, it is thought that cocoa flavanols (especially epicatechin) may have, in addition to antioxidant effects, action on enzymes such as eNOS and angiotensin-converting enzyme [17].
Dyslipidemia is a risk factor for cardiovascular disease, especially in people with T2DM. Alterations in lipid parameters trigger oxidative stress and influence insulin resistance and glycemic control. Three studies showed improvements in lipid profiles, which could be attributed to high-flavanol chocolate. In the study by Curtis, et al. [32], flavonoid administration resulted in additional improvements in lipoprotein status, particularly HDL and the TC/HDL ratio, despite all participants being treated with statins. However, it is noted that this effect may be due to certain flavonoid subclasses, and further studies are needed. In the study by Basu, et al. [25] employed acute cocoa supplementation with a high-fat meal, which may explain why positive results were seen only in HDL cholesterol, with no decrease in total cholesterol, TG, or LDL cholesterol, or in inflammatory markers. The two previous studies are not comparable because they used different doses of polyphenols and the intervention time was different (6 hours vs. 1 year). In the study by Mellor, et al. [30], after a 16-week follow-up, the group treated with high-polyphenol chocolate experienced significant improvements in HDL levels and TC/HDL ratio compared to the control group, although this reversed when the supplement was discontinued. Flavanols have demonstrated lipid-lowering effects in in vitro studies, acting as inhibitors of enzymes such as lipase, or in animal models, where a decrease in lipid accumulation in different organs and β cells has been observed, in addition to acting as reducers of plasma TG levels [16].
In summary, almost all studies show beneficial effects of cocoa flavonoids on some type of biochemical parameter. Although this food, along with other foods rich in polyphenols, could be used as chemopreventive agents against the development of diabetes and other cardiovascular diseases, studies to date do not offer entirely conclusive results.
Furthermore, industrialization and the current pace of life have meant that cocoa consumption is no longer limited to powdered forms or dark chocolate with a high percentage of cocoa. Instead, there are numerous foods containing cocoa, but with few or no beneficial properties: milk chocolate, white chocolate, cocoa cream, cocoa drinks, etc. This leads many people to include chocolate in their diet, but not the recommended type, which contains more than 70% cocoa. Instead, they include other varieties that are high in fats and sugars, and are even harmful to health. Therefore, more in vitro studies and animal models are needed to elucidate the mechanisms by which flavanols offer real benefits. These studies also include well-designed epidemiological and intervention studies in humans that take into account the amount and type of chocolate consumed, alone or in combination with the diet, to determine its impact on the development of T2DM.
Conclusion
Most of the studies reviewed over the last fifteen years conclude that daily, moderate, and long-term consumption of cocoa as part of a varied and healthy diet, alone or in combination with other foods rich in polyphenols, could be beneficial in preventing chronic diseases associated with aging, such as diabetes.
Although a substantial body of in vitro and animal model research supports the benefits of cocoa flavanols as antidiabetics (even exerting an effect similar to that of certain drugs such as metformin), the exact mechanisms that make these effects possible, as well as the doses that could be effective, are unknown, given their low bioavailability in the body.
All of this, combined with the disparity of results obtained in the scientific literature to date, necessitates further research to clarify the role of cocoa-enriched diets in diabetes management and evaluate the actions of pure compounds in isolation to establish which specific flavanols yield therapeutic benefits and at what dosage levels these effects are optimized.
For the reasons mentioned above, it remains premature to recommend routine cocoa consumption for diabetic populations until the existing doubts are resolved.
- International Diabetes Federation. IDF Diabetes Atlas, 9th edition [Internet]. 2019. Available from: https://www.diabetesatlas.org
- Rojo-Martínez G, Aguilera-Venegas G, Badía-Guillén R, Calle-Pascual A, Castaño L, Castell C, et al. Incidence of diabetes mellitus in Spain as results of the nation-wide cohort [email protected] study. Sci Rep. 2020 Feb 20;10(1):2765. Available from: https://doi.org/10.1038/s41598-020-59643-7
- National Institute of Statistics. Deaths by Cause (reduced list) by Sex and Age Groups [Internet]. 2018. Available from: https://www.ine.es/jaxiT3/Datos.htm?t=7947#!tabs-tabla. Accessed 2020 Jul 01.
- National Institute of Statistics. Population by Sex, Age, Chronic Disease, and Diagnosis [Internet]. 2018. Available from: https://www.ine.es/jaxi/Datos.htm?path=/t15/p419/p01/a2003/l0/&file=01011.px#!tabs-tabla. Accessed 2020 Jul 01.
- Del Rio D, Borges G, Crozier A, Rodríguez-Mateos A, Spencer JPE, Tognolini M. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid Redox Signal. 2013 May 20;18(14):1818-92. Available from: https://doi.org/10.1089/ars.2012.4581
- Kim Y, Clifton P, Keogh J. Polyphenols and Glycemic Control. Nutrients. 2016 Jan 11;8(1):17. Available from: https://doi.org/10.3390/nu8010017
- Castro del Pozo S, Pérez Arellano JL. Manual de patología general. Barcelona: Elsevier; 2019. 908 p.
- Porth Grossman SC, Porth CM. Porth physiopathology: health alterations, basic concepts. Spain: Wolters Kluwer; 2014. 1662 p. Available from: https://www.scirp.org/reference/referencespapers?referenceid=2215862
- Martin MA, Goya L, Ramos S. Antidiabetic actions of cocoa flavanols. Mol Nutr Food Res. 2016 Aug;60(8):1756-69. Available from: https://doi.org/10.1002/mnfr.201500961
- Marshall WJ, Bangert SK, Lapsley M. Clinical biochemistry. Spain: Elsevier; 2013. 384 p.
- Martin MA, Goya L, Ramos S. Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies. Food Chem Toxicol. 2017 Nov;109(Pt 1):302-14. Available from: http://doi.org/10.1016/j.fct.2017.09.015
- Fraga CG, Galleano M, Oteiza PI, Verstraeten SV. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010 Dec;31(6):435-45. Available from: https://doi.org/10.1016/j.mam.2010.09.006
- Bernatoniene J, Kopustinskiene D. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018 Apr 20;23(4):965. Available from: https://doi.org/10.3390/molecules23040965
- Al-Ishaq RK, Abotaleb M, Büsselberg D, Kajo K, Kubatka P. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules. 2019 Sep 03;9(9):430. Available from: https://doi.org/10.3390/biom9090430
- Babu PVA, Gilbert ER, Liu D. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013 Nov;24(11):1777-89. Available from: https://doi.org/10.1016/j.jnutbio.2013.06.003
- Strat K, Davy B, Davy KP, Hulver M, Liu D, Neilson AP, et al. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J Nutr Biochem. 2016 Sep;35:1-21. Available from: https://doi.org/10.1016/j.jnutbio.2015.12.008
- Osakabe N, Terao J. Possible mechanisms of postprandial physiological alterations following flavan 3-ol ingestion. Nutr Rev. 2018 Mar 01;76(3):174-86. Available from: https://doi.org/10.1093/nutrit/nux070
- Greenberg JA, Garcia L, Manson JE, Neuhouser ML, Philips LS, Tinker L, et al. Chocolate intake and diabetes risk in postmenopausal American women. Eur J Clin Nutr. 2017 Sep;71(9):1088-93. Available from: https://doi.org/10.1038/ejcn.2017.36
- Matsumoto C, Djoussé L, Gaziano JM, Petrone AB, Sesso HD. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015 Feb;101(2):362-7. Available from: https://doi.org/10.3945/ajcn.114.092221
- Zamora-Ros R, Amiano P, Buijsse B, Boeing H, Bredsdorff L, Fagherazzi G, et al. Dietary Intakes of Individual Flavanols and Flavonols Are Inversely Associated with Incident Type 2 Diabetes in European Populations. J Nutr. 2014 Mar;144(3):335-43. Available from: https://doi.org/10.3945/jn.113.184945
- Oba S, Fujii K, Kawachi T, Nagata C, Nakamura K, Shimizu H, et al. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br J Nutr. 2010 Feb;103(3):453-9. Available from: https://doi.org/10.1017/s0007114509991966
- Crichton GE, Dearborn P, Elias M, Robbins M. Habitual chocolate intake and type 2 diabetes mellitus in the Maine-Syracuse Longitudinal Study: (1975–2010): Prospective observations. Appetite. 2017 Jan 01;108:263-9. Available from: https://doi.org/10.1016/j.appet.2016.10.008
- Maskarinec G, Boushey CJ, Jacobs S, Haiman CA, Kolonel LN, Le Marchand L, et al. Intake of Cocoa Products and Risk of Type 2 Diabetes: The Multiethnic Cohort. Eur J Clin Nutr. 2019 May;73(5):671-8. Available from: https://doi.org/10.1038/s41430-018-0188-9
- Greenberg JA. Chocolate intake and diabetes risk. Clin Nutr. 2015 Feb;34(1):129-33. Available from: https://doi.org/10.1016/j.clnu.2014.02.005
- Basu A, Aston CE, Betts NM, Fu D, Leyva MJ, Lyons TJ. Acute Cocoa Supplementation Increases Postprandial HDL Cholesterol and Insulin in Obese Adults with Type 2 Diabetes after Consumption of a High-Fat Breakfast. J Nutr. 2015 Oct;145(10):2325-32. Available from: https://doi.org/10.3945/jn.115.215772
- Rynarzewski J, Dicks L, Ellinger S, Helfrich HP, Ludwig N, Stoffel-Wagner B, et al. Impact of a Usual Serving Size of Flavanol-Rich Cocoa Powder Ingested with a Diabetic-Suitable Meal on Postprandial Cardiometabolic Parameters in Type 2 Diabetics—A Randomized, Placebo-Controlled, Double-Blind Crossover Study. Nutrients. 2019 Feb 19;11(2):417. Available from: https://doi.org/10.3390/nu11020417
- Mellor DD, Atkin SL, Kilpatrick ES, Madden LA, Smith KA. High-polyphenol chocolate reduces endothelial dysfunction and oxidative stress during acute transient hyperglycaemia in Type 2 diabetes: A pilot randomized controlled trial. Diabet Med. 2013 Apr;30(4):478-83. Available from: https://doi.org/10.1111/dme.12030
- Balzer J, Gross BH, Heiss C, Heussen N, Keen CL, Kelm M, et al. Sustained Benefits in Vascular Function Through Flavanol-Containing Cocoa in Medicated Diabetic Patients: A Double-Masked, Randomized, Controlled Trial. J Am Coll Cardiol. 2008 Jun 03;51(22):2141-9. Available from: https://doi.org/10.1016/j.jacc.2008.01.059
- Dicks L, Ellinger S, Gronwald D, Helfrich H-P, Kirch N, Wernken K, et al. Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension - A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients. 2018 Oct 06;10(10):1435. Available from: https://doi.org/10.3390/nu10101435
- Mellor DD, Atkin SL, Beckett S, Kilpatrick ES, Sathyapalan T, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet Med. 2010 Nov;27(11):1318-21. Available from: https://doi.org/10.1111/j.1464-5491.2010.03108.x
- Ramírez-Sánchez I, Ceballos G, Ciaraldi TP, Coe T, Henry R, Hogan M, et al. (−)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int J Cardiol. 2013 Oct 09;168(4):3982-90. Available from: https://doi.org/10.1016/j.ijcard.2013.06.089
- Curtis PJ, Cassidy A, Dhatariya K, Kroon PA, Potter J, Sampson M. Chronic Ingestion of Flavan-3-ols and Isoflavones Improves Insulin Sensitivity and Lipoprotein Status and Attenuates Estimated 10-Year CVD Risk in Medicated Postmenopausal Women with Type 2 Diabetes. Diabetes Care. 2012 Feb;35(2):226-32. Available from: https://doi.org/10.2337/dc11-1443
- Rostami A, Ebrahimpour-Koujan S, Eghtesadi M, Eghtesadi S, Haghighat N, Heidari I, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015 Jan;11(1):21-9. Available from: https://pubmed.ncbi.nlm.nih.gov/26089927/
- Haghighat N, Eghtesadi S, Heidari I, Hoseini A, Rostami A, Shidfar F. The effects of dark chocolate on glycemic control and blood pressure in hypertensive diabetic patients: a randomized clinical trial. Razi J Med Sci. 22013 [cited 2025 Jun 30];20(113):78-86.
- Fernández-Millán E, Álvarez C, Cordero I, Escrivá F, Goya L, Martín MA, et al. Cocoa‐rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol Nutr Food Res. 2015 Apr;59(4):820-4. Available from: https://doi.org/10.1002/mnfr.201400746
- Martín MA, Ramos S. Cocoa polyphenols in oxidative stress: Potential health implications. J Funct Foods. 2016 Dec;27:570-88. Available from: https://doi.org/10.1016/J.JFF.2016.10.008
- Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr. 2013;33:105-28. Available from: https://doi.org/10.1146/annurev-nutr-071811-150642
- Organización Mundial de la Salud (OMS). Diabetes [Internet]. 2025. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1. Accessed 2025 Jun 30.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
